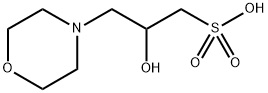
Synthesis and Application of 3-(N-Morpholino)-2-hydroxypropanesulfonic acid (MOPSO)
MOPSO (3-(N-Morpholino)-2-hydroxypropanesulfonic acid), a novel zwitterionic buffer employed in biological research, exhibits a buffering range of pH 6.2 to 7.6. This compound is primarily utilized in biochemical studies as an advanced zwitterionic buffering agent for biological systems.
1. The Application of MOPSO
MOPSO is the most widely used in various biological and biochemical studies due to its presumably more inert properties.[1] Mohamed Taha and co-workers found that because MOPSO has one more OH group, this group interacts with water clusters around the hydrophobic isopropyl groups, which changes the hydration state of the polymer. It should also be noted that the phase transition in the presence of buffers is similar to those observed with kosmotropic salts and sugars (protein stabilizers) in terms of lowering the LCST and the dehydration of PNIPAM groups. Such favoring of the compact globule state observed at high buffer concentrations will provide a protective effect against denaturation of globular proteins. Studies investigating the interactions of MOPSO buffers with bovine serum albumin (BSA) have found that these buffers stabilize BSA.[2]

Fig.1. MOPSO
Liquid formulation is the preferred presentation for diagnostic reagents, making the maintenance of liquid-state stability a primary concern. The use of MOPSO buffer, either employed independently or in combination with phosphate buffer, preserves enzymatic activity following freeze-thaw cycles. Consequently, this approach ensures the stability of liquid reagents.[3]
MOPSO also finds application in the domain of solar cells. Poly(3,4-ethylene dioxythiophene)poly(styrene sulfonate) (PEDOT:PSS) was doped by a novel zwitterion, 3-(N-morpholino)-2-hydroxypropanesulfonic acid (MOPSO), leading to a dramatic improvement in its conductivity and consequently enhanced efficiency in polymer solar cells (PSCs) based on PEDOT:PSS hole transport layer (HTL) and versatile photoactive systems.[4]
2. Synthetic Method of 3-(N-Morpholino)-2-hydroxypropanesulfonic Acid(MOPSO)
Synthesis Method 1:
A mixture of 174.24 g (2 moles) of morpholine, 300 mL of water, and 196.5 g (1 mole) of sodium 3-chloro-2-hydroxypropyl sulfonate was added to a 1-liter, 2-neck flask equipped with a stirrer and condenser. The suspension was stirred vigorously and refluxed for 3 hours. After cooling to about 25°C, the solution was passed through a column of Dowex 50 to remove sodium cations and form the free acid. The eluate from the column, containing the product, was evaporated to a thick oil under vacuum. Upon diluting with alcohol, the product, in the form of a zwitterion, crystallized readily. The product was filtered, washed with alcohol, and dried. to obtain MOPSO. The yield was 160 g of a white material melting at 280°C with decomposition. Elemental analysis indicated carbon content of 37.38%, hydrogen content of 6.74%, and nitrogen content of 6.24%. The calculated values are carbon, 37.3%; hydrogen, 6.7%; and nitrogen, 6.2%. The pKa of this product at 20°C is 6.95.[5]
Synthesis Method 2:
Diethanolamine was added to the reactor and heated to 55°C, followed by dropwise addition of concentrated sulfuric acid. After completion of the addition, the temperature was raised to 200°C, and sulfuric acid along with a catalyst mixture of Ni, Cu, and MoO₃ (mass ratio = 1:3:4) was introduced. The reaction was stirred for 70 minutes. Upon completion, the mixture was cooled to 60°C and neutralized with 1 mol/L aqueous sodium hydroxide solution, then stirred for an additional 20 minutes. The neutralized reaction mixture was cooled to room temperature, filtered to remove sodium sulfate, and mixed with 1.5 equivalents of 45% aqueous sodium hydroxide solution under specified reaction conditions followed by fractional distillation with fractions collected at overhead temperatures of 130℃ and 160℃, respectively; to the fractionated liquid, sodium 2-hydroxypropanesulfonate was added at a morpholine-to-sodium 2-hydroxypropanesulfonate molar ratio of 1:1.2, and the mixture was stirred under reflux for 3 hours; after cooling to room temperature, ion-exchange chromatography was performed using a strongly acidic styrene-based cation exchange resin; subsequent elution with 5% aqueous ammonia solution, vacuum concentration, pH adjustment to 5.0 with glacial acetic acid, and dilution with a ten-fold volume of ethanol afforded a white precipitate, which was isolated by filtration, washed with ethanol, and dried under vacuum to get MOPSO.[6]
Synthesis Method 3:
87 g of morpholine was added to a 1 L three-necked flask, stirred while heating to 60℃; subsequently, 2 g of anhydrous sodium carbonate was added and maintained at this temperature with stirring for 20 min; 98 g of 3-chloro-2-hydroxypropanesulfonic acid sodium salt was weighed and added in three portions, with a slight exotherm observed during addition; after completion of addition, the temperature was raised to 95℃ and stirred for 2 h; upon reaction completion, heating was discontinued, the mixture cooled to 60℃, and 20 g of hydrochloric acid added dropwise to adjust pH to 4.5–5, followed by filtration to collect Filter Cake I and filtrate; the filtrate was cooled to 5–10℃ and crystallized for 2 h, then suction-filtered to obtain Filter Cake II; Filter Cakes I and II were combined and dried to afford crude 3-(N-morpholino)-2-hydroxypropanesulfonic acid. The crude product was added to 300 mL methanol, stirred under reflux, supplemented with 100 mL water until complete dissolution, treated with 1.0 g activated carbon for decolorization while maintaining temperature for 1 h; after hot filtration, the filtrate was cooled to 5℃ and crystallized for 2 h; the resultant solid was suction-filtered and dried at 55℃ in a forced-air oven for 4 h to yield 93.75 g of MOPSO (83.3% yield).[7]
References
[1]Zhao, Guanghua, Oxidation of Good's buffers by hydrogen peroxide, [J]Analytical Biochemistry (2006), 349(2), 262-267.
[2]Mohamed Taha, Interactions of Biological Buffers with Macromolecules:The Ubiquitous "Smart" Polymer PNIPAM and the Biological Buffers MES, MOPS, and MOPSO, [J]Macromolecules (Washington, DC, United States) (2011), 44(2), 8575-8589.
[3]Randox Laboratories Ltd, Improved stability of glucose oxidase and horseradish peroxidase to freezing and thawing using zwitterionic buffers, [P] EP1199357.
[4]Zhiqiang Zhao, Efficiency enhancement of polymer solar cells via zwitterion doping in PEDOT:PSS hole transport layer, [J] Organic Electronics (2015), 27, 232-239.
[5]Research Organics, Aminohydroxyalkylsulfonic acid zwitterions, [P]US4169950.
[6]Suqian Yuanyang Biotechnology Co., Ltd., Process for synthesizing 3-(N-morpholine)-2-hydroxy propanesulfonic acid, [P]CN106117163A.
[7]Jining Yake New Material Technology Co., Ltd., Solvent-free synthetic method of 3-(N-morpholimyl)-2-hydroxypropane sulfonic acid (MOPSO) from morpholine and sodium 3-chloro-2-hydroxypropane sulfonate, and application thereof as zwitterionic buffer for bioresearch, [P]CN113620908B.
You may like
See also
Lastest Price from MOPSO manufacturers

US $0.00/KG2025-04-21
- CAS:
- 68399-77-9
- Min. Order:
- 1KG
- Purity:
- 99%-101%
- Supply Ability:
- 100KG

US $0.00-0.00/kg2025-04-21
- CAS:
- 68399-77-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20MT