Synthesis and application of 2-Chloro-5-Nitropyridine
Introduction
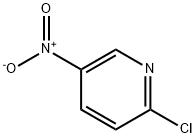
2-Chloro-5-Nitropyridine is a light white to yellow solid powder at room temperature.(Figure 1) It is insoluble in water and soluble in organic solvents such as ether and carbon tetrachloride. The nitro group on the pyridine ring has halogen like properties and is easily replaced by nucleophiles. 2-Chloro-5-Nitropyridine is an important pyridine derivative, which is developed and applied as fungicides, plant growth regulators, as well as pharmaceutical intermediates.

Synthesis of 2-Chloro-5-Nitropyridine from Report 1
Researching on the synthesis 2-Chloro-5-Nitropyridine is of great practical value. According to the reference and mechanism analysis, several possible synthesis methods were designed in this dissertation. After a full evaluation, a best synthetic route was determined: Started from 2-Aminopyridine, which was nitrified by mixed acid of HNO3; and H2SO4, 2-Amino-5-Nitropyrodine was prepared. Then, under the acidic condition, as a result of diazotization and hydrolysis reaction, 2-Hydroxy-5-Nitropyridine was prepared. At last, 2-Hydroxy-5-Nitropyridine was chlorinated by PCl5/POCl3 to be 2-Chloro-5-Nitropyridine. The total yield of 2-Chloro-5-Nitropyridine is 41.1 %.[1]
Synthesis of 2-Chloro-5-Nitropyridine from Report 2
A mixture of Potassium 5-nitropyridine-2-sulfonate (2a) (2.00 mmol)and PCl5 (4.17 g, 20.00 mmol) was heated to reflux for 0.5h. Xylene (2×10ml) was added and the mixture distilled under reduced pressure to remove excess PCl5. The residue was purified by flash chromatography (50 ml silica, 2.5 cm column diameter, eluant: pentane-CH2Cl2=60:40). This gave 2-chloro-5-nitropyridine as a white solid (275 mg, 87% yield) with mp 110.0-111.5℃ (lit.109℃). The spectroscopic properties were the same as for commercial 2-chloro-5-nitropyridine.[2]
Application Researches
Ring opening of pyridines
During the 1980s, Reinheimer et al. (1980, 1984) investigated the reaction of 2-chloro-5-nitropyridine with an excess of sodium hydroxide in dimethyl sulfoxide/water solutions. Using visible spectroscopy, in addition to 1H-NMR and 13C-NMR, they were able to show that a relatively stable intermediate was formed. They proposed that the pseudo-cis isomer, (I), would enable the intermediate to undergo the second part of the reaction sequence, namely ring closure. This reaction sequence was named by van der Plas (1978) as the SN(ANRORC) process (addition of the nucleophile, ring opening and ring closure).
The reaction of 2-chloro-5-nitropyridine with two equivalents of base produces the title carbanion as an intermediate in a ring-opening/ring-closing reaction. The crystal structures of the tetra-n-butylammonium salts of the intermediates, C16H36N+·C5H3N2O3-, revealed that pseudo-cis and pseudo-trans isomers are possible. One crystal structure displayed a mixture of the two isomers with approximately 90% pseudo-cis geometry and confirms the structure predicted by the SN(ANRORC) mechanism. The pseudo-cis intermediate undergoes a slow isomerization over a period of months to the pseudo-trans isomer, which does not have the appropriate geometry for the subsequent ring-closing reaction. The structure of the pure pseudo-trans isomer is also reported. In both isomers, the negative charge is highly delocalized, but relatively small differences in C-C bond distances indicate a system of conjugated double bonds with the nitro group bearing the negative charge. The packing of the two unit cells is very similar and largely determined by the interactions between the planar carbanion and the bulky tetrahedral cation.[3]
Synthesis of phenylpiperazine derivatives
Novel thiourea (5a, 5b) and thiazolidinone derivatives (6a, 6b) were synthesized by hybridizing molecules starting from the compound 6-(4-phenylpiperazin-1-yl)pyridin-3-amine (4) which is known to show anticancer activity. The synthesis of the leading compound was carried out by using 1-(5-nitropyridin-2-yl)-4-phenylpiperazine (3) which was obtained by a novel method of the reaction of 2-chloro-5-nitropyridine (1) and N-phenylpiperazine (2). The structures of the compounds were confirmed using FTIR, 1H-NMR, 13C-NMR, HRMS spectroscopic methods and elemental analysis. The organic molecules were tested for their anticancer activities against prostate cancer (PC) cell lines: DU145, PC-3 and LNCaP. As the compound 5a exerted the highest cytotoxic activity, IC50 concentrations of compound 5a were further investigated in terms of morphology, colony-forming ability, RNA expression, fragmented DNA and cell cycle distributions of PC cell lines. Overall data revealed that compound 5a treatment induces apoptosis and DNA fragmentation in prostate cancer cell lines and inhibits cell cycle progression resulting in the accumulation of cells in either the G1 or the S phases.[4]
References
1.Shao YX.Synthesis of 2-chloro-5-nitropyridine and 4-amino-2-chloropyridine[D].Nanjing University of Science & Technology,2009.
2.Bakke JM, Sletvold I. Substitution reactions of 5-nitropyridine-2-sulfonic acid. A new pathway to 2,5-disubstituted pyridines. Org Biomol Chem. 2003;1(15):2710-2715. doi:10.1039/b305620a
3.Zeller M, Pett VB, Haynes LW. Ring opening of pyridines: the pseudo-cis and pseudo-trans isomers of tetra-n-butylammonium 4-nitro-5-oxo-2-pentenenitrilate. Acta Crystallogr C. 2007;63(Pt 6):o343-o346. doi:10.1107/S0108270107019506
4.Demirci S, Hayal TB, Kıratlı B, et al. Design and synthesis of phenylpiperazine derivatives as potent anticancer agents for prostate cancer. Chem Biol Drug Des. 2019;94(3):1584-1595. doi:10.1111/cbdd.13575
Lastest Price from 2-Chloro-5-nitropyridine manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 4548-45-2
- Min. Order:
- 1KG
- Purity:
- 98%min HPLC
- Supply Ability:
- 1000KGS

US $0.00/KG2025-04-21
- CAS:
- 4548-45-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt