Summary of fluoroiodomethane uses
Description
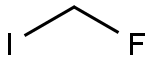
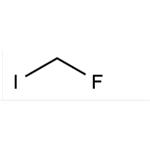
In recent years, fluoroiodomethane (CH2FI) has emerged as an easy-to-handle, non-ozone-depleting agent and readily available platform for monofluoromethylation strategies. Recent applications in nucleophilic substitutions, lithiation reactions, transition-metal catalyzed transformations, radical processes, and 18F-radiolabelling chemistry showcase this reagent's potential for preparing organofluorine compounds.
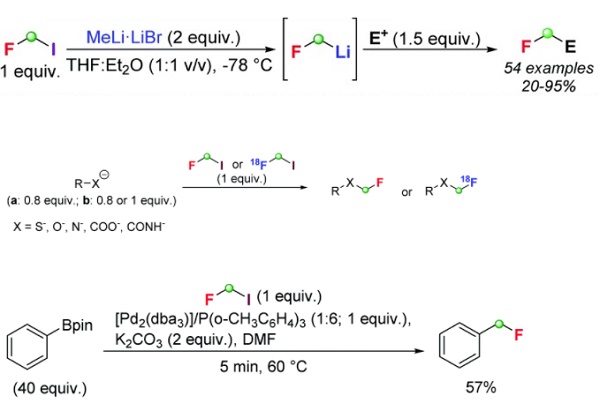
Use as a precursor of fluorinated lithium carbenoids.
In principle, a nucleophilic installation of the monofluoromethyl group could be achieved by directly adding a fluoromethyl carbanion to a suitable electrophile. However, the remarkable chemical instability of fluorinated carbanions limited this strategy. fluoroiodomethane has emerged as a suitable C1 building block for generating metallated fluorinated species. Indeed, CH2FI holds two possible metalation sites, conceptually leading to M–CH2–F or M–CHI–F – i.e., fluorinated carbenoids (M = metal) – through metal/iodine or metal/hydrogen exchange reactions, respectively.
In 2017, researchers provided the first and still unique example of a direct nucleophilic monofluoromethylation protocol exploiting CH2FI as the precursor of fluoromethyllithium. The researcher was generated using MeLi·LiBr as a lithiation agent in a 1 : 1 mixture of THF/Et2O in the presence of the electrophilic partner (Barbier-type conditions) at −78 °C. The robustness of the strategy was demonstrated by reporting the preparation of more than 45 monofluoromethylated adducts employing aldehydes, ketones, imines, and Weinreb amides as electrophilic partners.
Use as an electrophilic reagent.
The electron-withdrawing effect of the fluorine atom on the carbon center makes, in principle, fluoroiodomethane a potential electrophilic platform prone to undergo substitution reactions through an SN2 mechanism. Several nucleophiles have been employed in reactions with CH2FI affording the corresponding fluoromethylated adducts. Furthermore, the possibility of access to radiolabelled fluoroiodomethane, i.e. [18F]fluoroiodomethane, paved the way for applications of fluoromethylated compounds in positron emission tomography (PET).
Use in transition-metal catalyzed monofluoromethylation
Fluoroiodomethane has found applications in transition metal-catalyzed monofluoromethylations. To the best of our knowledge, the first example of this kind of transformation was reported by Suzuki. This author described a Csp3–Csp2 type Suzuki–Miyaura coupling involving the reaction of CH2FI with a large excess (40 equivalents) of pinacol phenylboronate in the presence of Pd2(dba)3/P(o-CH3C6H4)3 (1 : 6), affording fluoromethylbenzene in 57% yield.
References
[1] Marco Colella . “The synthetic versatility of fluoroiodomethane: recent applications as monofluoromethylation platform.” Organic Biomolecular Chemistry 20 23 (2022): Pages 4669-4680.
You may like
See also
Lastest Price from Fluoroiodomethane manufacturers
US $1.00/KG2024-06-13
- CAS:
- 373-53-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20T