Sodium triacetoxyborohydride: Applications in Selective Reductive Amination and its Detection Method
General Description
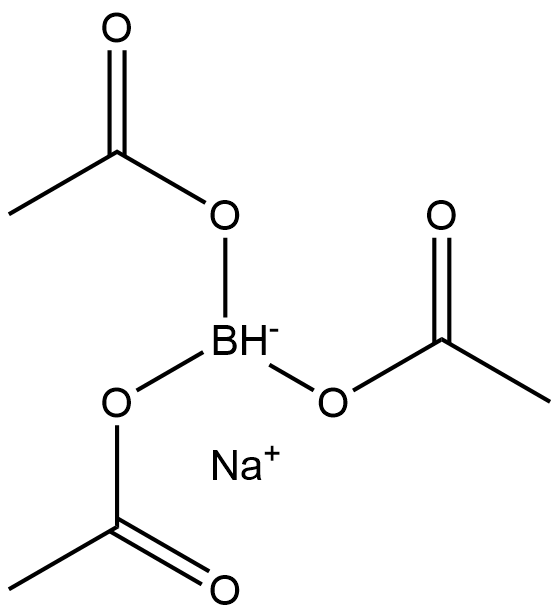
Sodium triacetoxyborohydride, or sodium triacetoxyhydroborate or STAB, is a chemical compound with the formula Na[(CH3COO)3BH]. Like other borohydrides, it is a reducing agent in organic synthesis. This colourless salt is prepared by protonolysis of sodium borohydride with acetic acid.

It is a mild reducing agent that is commonly used in reductive aminations. STAB has the advantage over sodium cyanoborohydride (NaBH3CN) because it does not produce toxic side-pdts. One disadvantage of STAB is that it is H2O sensitive, not compatible with MeOH, and reacts slowly with EtOH and isopropanol. For these reasons, STAB is typically used with aprotic solvents such as DCE, DCM, THF, dioxane, or toluene.
Reductive amination
Reductive amination is one of the most important methods to synthesize amines, having a wide application in the pharmaceutical, agricultural, and materials industries. Regarding pharmaceuticals, nearly one-fourth of all C−N bond-forming reactions are performed via reductive amination[1]. The reaction begins with dehydration between a carbonyl compound and an amine compound to form an imine, which is then reduced to an alkylated amine product. Direct reductive amination, wherein the imine formation and subsequent reduction occur in situ, presents a convenient one-pot synthesis method to produce alkylated amines. Under these reaction conditions, the choice of reducing agent is crucial as it must selectively reduce the imine over the carbonyl compound starting material.
Sodium triacetoxyborohydride (STAB) is one of the primary reducing agents utilized for these reactions, as it can selectivity enable direct reductive amination of imines from aldehyde and ketone starting materials without competing reduction of the carbonyl functional group. STAB has a much-improved safety profile over similar selective reducing agents such as sodium cyanoborohydride. Though examples of alternate conditions have been developed for large-scale reductive amination processes, STAB continues to be one of the most widely used reagents to perform reductive amination chemistry[2].
The selectivity of STAB for imines has been exploited in many synthesis protocols, such as within drug patents for cinacalcet, lapatinib, and pramipexole, which all use reductive amination between an aldehyde and a primary amine at one step of their synthesis. The selectivity exhibited by STAB is postulated to be attributed to the three acetoxy groups, as they can stabilize the B−H bond via steric shielding and electron-withdrawing effects.
Detection method
As a common reducing agent, its potency degrades over time and is not uniformly assigned. A simple assay based on an aldehyde reduction has been developed to determine the active borohydride content of this reagent. The HPLC assay yield of a salicylaldehyde reduction has been shown to accurately determine this potency. It has been validated against the H2 evolution method and yields obtained from a reductive amination. The use of these assay data to adjust the STAB charge and optimize a reductive amination has been demonstrated[3].
In another method, researchers report the development and qualification of a rapid, quantitative GC derivatization method using 3,4-dihydroisoquinoline to enable accurate hydride assay determination of STAB. Several STAB samples were analyzed using both GC and HPLC methods. Additionally, Raman spectroscopy was evaluated to probe the degradation kinetics of STAB, where samples were monitored in an open container throughout 60 h. Data obtained using Raman is advantageous in that the rapid sampling rate enables the construction of a continuous plot of STAB degradation over a 16 h period[4].
References:
[1] SHANNON J. OLIPHANT. Density Functional Theory Study on the Selective Reductive Amination of Aldehydes and Ketones over Their Reductions to Alcohols Using Sodium Triacetoxyborohydride[J]. ACS Omega, 2022, 7 34: 29526-30656. DOI:10.1021/acsomega.2c04056.[2] IAN HALE R W James Chadwick. Quantitative GC determination of sodium triacetoxyborohydride (STAB)[J]. Journal of pharmaceutical and biomedical analysis, 2021, 203. DOI:10.1016/j.jpba.2021.114213.
[3] MICHAEL J. ZACUTO* R D Joseph Perona. A Quantitative Assay of Sodium Triacetoxyborohydride[J]. Organic Process Research & Development, 2019, 23 9: 1773-2106. DOI:10.1021/acs.oprd.9b00215.
Lastest Price from Sodium triacetoxyborohydride manufacturers

US $1.00/g2025-04-21
- CAS:
- 56553-60-7
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg
US $10.00/KG2025-04-21
- CAS:
- 56553-60-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt