Sodium Stearyl Fumarate: An Essential Lubricant in Solid Dosage Forms with Safety Considerations
General Description
Sodium Stearyl Fumarate is a widely used lubricant in the manufacturing of solid dosage forms, providing several advantages over other lubricants such as magnesium stearate and talc. However, a recent study highlighted a potential incompatibility issue between Sodium Stearyl Fumarate and secondary amines, which could lead to the formation of degradation products. To minimize this reaction, it is recommended to optimize the microenvironment by adjusting the pH and incorporating moisture protection measures. While Sodium Stearyl Fumarate is generally considered safe for use, precautions should be taken to avoid direct contact with the skin and eyes, and to follow proper handling guidelines. Overall, Sodium Stearyl Fumarate is an essential component in the manufacturing of high-quality solid dosage forms when handled properly and with careful consideration of formulation design and stability considerations.

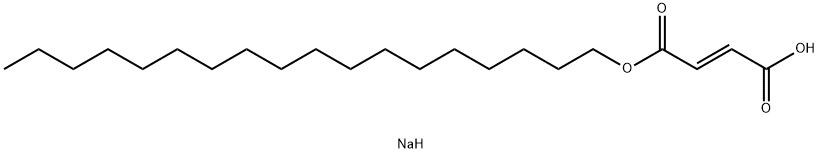
Figure 1. Sodium stearyl fumarate
Properties as a Lubricant
Sodium Stearyl Fumarate is a type of lubricant that is used in the manufacturing of solid dosage forms. Lubricants play a crucial role in reducing interparticle friction, facilitating the ejection of tablets from die cavities and enhancing the flow rate of tablet granulates. Compared to other lubricants such as magnesium stearate and talc, Sodium Stearyl Fumarate has several advantages. It is an inert and hydrophilic lubricant, which solves compatibility issues with magnesium stearate. Additionally, Sodium Stearyl Fumarate provides superior hardness at equivalent compression forces, and has less impact on disintegration time. Sodium Stearyl Fumarate is also semi-soluble, thereby resulting in low residue in solution or effervescent preparations. Overall, Sodium Stearyl Fumarate is an excellent substitute for magnesium stearate and talc. The typical amount of Sodium Stearyl Fumarate used is in the range of 0.25 to 3% w/w. Its unique properties make it an essential component in the manufacturing of high-quality solid dosage forms. 1
Applications in the Drug Product
Sodium Stearyl Fumarate is commonly used as an excipient in pharmaceutical formulations. However, a compatibility study of the AZD7986 project uncovered a potential issue with tablets containing Sodium Stearyl Fumarate under humid conditions. It was observed that regardless of the choice of disintegrant or filler combination, a degradation product of the active pharmaceutical ingredient (API) appeared at the tail of the chromatogram. Further analysis using high resolution mass spectrometry revealed that this degradant was a Michael addition product of the API and fumaric acid. To confirm the proposed structure and reaction mechanism, a reaction between deuterated fumaric acid and the API was conducted. This validated the formation of the identified degradant. It was also discovered that fumaric acid itself was a degradation product of PRUV in the presence of other excipients, indicating a possible hydrolysis reaction. Importantly, the Michael addition reaction was found to be facilitated by water and basic conditions. This study serves as a precautionary note for projects utilizing Sodium Stearyl Fumarate as an excipient, particularly when the API can act as a nucleophile. To minimize the reaction, it is recommended to optimize the microenvironment by adjusting the pH and incorporating moisture protection measures. This newly discovered incompatibility between Sodium Stearyl Fumarate and secondary amines highlights the importance of considering formulation design and stability considerations when using Sodium Stearyl Fumarate in drug products. 2
Safety Considerations
Sodium Stearyl Fumarate is generally considered to be safe for use in various applications. However, it is important to handle this substance with caution as it may cause skin and eye irritation. Direct contact with the skin can lead to redness, itching, and inflammation. In case of eye exposure, it may cause severe irritation, redness, and discomfort. Therefore, it is recommended to wear protective gloves and goggles when working with Sodium Stearyl Fumarate. While skin and eye irritation are the main safety concerns associated with Sodium Stearyl Fumarate, there is limited evidence suggesting any significant systemic toxicity or long-term health effects. It is important to note that the safety of Sodium Stearyl Fumarate largely depends on its proper handling and adherence to safety guidelines. In the event of accidental ingestion or prolonged exposure, it is advised to seek medical attention immediately. It is also crucial to follow good manufacturing practices and employ appropriate safety measures, such as adequate ventilation and personal protective equipment, to minimize the risk of adverse effects. Overall, Sodium Stearyl Fumarate is considered safe when handled properly, but precautions should be taken to avoid direct contact with the skin and eyes. 3
Reference
1. Miller T, York P. Pharmaceutical tablet lubrication, International Journal of Pharmaceutics. 1988, 41: 1-19.
2. Ludvigsson JW, Wikström H, Andersson T, Norrby PO. Degradation caused by incompatibility between sodium stearyl fumarate (PRUV) and AZD7986 in the drug product. J Pharm Biomed Anal. 2018, 158: 82-87.
3. Sodium octadecyl fumarate. European Chemicals Agency, EC/List no. 223-781-1.
You may like
Related articles And Qustion
See also
Lastest Price from Sodium Stearyl Fumarate manufacturers

US $0.00-0.00/kg2025-07-28
- CAS:
- 4070-80-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20MT

US $2.00-5.00/kg2025-07-08
- CAS:
- 4070-80-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg