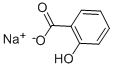
Sodium salicylate:Activity, Application and Chemical Studies
General Description
Sodium Salicylate Sodium Salicylate is a NF-κB inhibitor that decreases inflammatory gene expression and improves repair in aged muscle.[3] It can inhibits cyclo-oxygenase-2 (COX-2) activity independently of transcription factor (NF-κB) activation.[2]Sodium Salicylate is also a S6K inhibitor.Sodium salicylate has a wide range of uses, especially as an intermediate of antipyretic and analgesic drug aspirin, which has attracted widespread attention.[1]

Figure 1 Powder of the Sodium Salicylate
Preparation
The method for efficient production of sodium salicylate:First, the gas inlet and outlet of the upper part of the reactor 1 are filled with nitrogen gas into the reactor to a slight positive pressure through the nitrogen stamping device and the detachable pipeline, and 2.5kg of material is fed through the feeding port, and the moisture content of the material is 2.8%. Vacuumize the reactor to a vacuum degree of -0.93bar, then fill it with nitrogen to 2.0bar, repeat twice, turn on the stirring, turn on the heat conduction oil heating system, start heating up, the temperature of the material is raised to 140°C, and the CO2 inlet at the bottom of the reactor is at The operating gas velocity of 0.4m/s is used to feed CO2 to the reactor internal pressure of 0.45MPa. While maintaining the reaction temperature and reaction pressure, CO2 is discharged from the gas inlet and outlet of the upper part of the reactor. During the process, sodium phenate particles and CO2 undergo carboxylation reaction. Generate sodium salicylate particles, stop feeding CO2 after 30 minutes of reaction, release the pressure of the reactor to a slight positive pressure, then evacuate, stop heating, keep stirring open, press the reactor to 2.5MPa, open the material outlet to discharge, and discharge 2.86kg.
Second, the gas inlet and outlet of the upper part of the reactor 1 are filled with nitrogen gas into the reactor to a slight positive pressure through the nitrogen stamping device and the detachable pipeline, and 4.3kg of material is fed through the feeding port, and the moisture content of the material is 1.6%. Vacuumize the reactor to a vacuum degree of -0.93bar, then fill it with nitrogen to 2.0bar, repeat twice, turn on the stirring, turn on the heat conduction oil heating system, start heating up, the temperature of the material is raised to 140°C, and the CO2 inlet at the bottom of the reactor is at The operating gas velocity of 0.4m/s is used to feed CO2 to the reactor internal pressure of 0.65MPa. While maintaining the reaction temperature and reaction pressure, CO2 is discharged from the gas inlet and outlet of the upper part of the reactor. During the process, sodium phenate particles and CO2 undergo carboxylation reaction. Generate sodium salicylate particles, stop feeding CO2 after 50 minutes of reaction, release the pressure of the reactor to a slight positive pressure, then evacuate, stop heating, keep stirring open, press the reactor to 2.5MPa, open the material outlet to discharge, and discharge 4.97kg.[1]
Activity
In Vitro, sodium Salicylate is an effective inhibitor of COX-2 activity at concentrations far below those required to inhibit NF-κB (20 mg/mL) activation. Sodium Salicylate inhibits prostaglandin E2 release when add together with interleukin 1β for 24 hr with an IC50 value of 5 μg/mL, an effect that is independent of NF-κB activation or COX-2 transcription or translation. Sodium Salicylate acutely (30min) also causes a concentration-dependent inhibition of COX-2 activity measured in the presence of 0, 1, or 10μM exogenous arachidonic acid. In contrast, when exogenous arachidonic acid is increased to 30 μM, Sodium Salicylate is a very weak inhibitor of COX-2 activity with an IC50 of 100 μg/mL. When added together with IL-1β for 24hr, Sodium Salicylate causes a concentration-dependent inhibition of PGE2 release with an apparent IC50 value of approximately 5 μg/mL. The ability of Sodium Salicylate to directly inhibit COX-2 activity in A549 cells is tested after a 30-min exposure period, followed by the addition of different concentrations of exogenous arachidonic acid (1, 10, and 30μM). Sodium Salicylate causes a concentration-dependent inhibition of COX-2 activity in the absence of added arachidonic acid or in the presence of 1 or 10μM exogenous substrate with an apparent IC50 value of approximately 5 μg/mL.[4]
In Vivo, In C57Bl/6 DIO mice, Salicylate decreases both fasting and postprandial plasma glucose levels. Furthermore, there is a trend to reduce plasma triglyceride levels after Salicylate treatment in C57Bl/6 DIO mice (P=0.059). Salicylate significantly reduces 11β-HSD1 mRNA in omental adipose tissue in C57Bl/6 DIO mice, with a similar trend in mesenteric adipose (P=0.057). In mesenteric adipose of C57Bl/6 DIO mice, Salicylate also reduces 11β-HSD1 enzyme activity.[4]
Application
On the one hand, Sodium salicylate can reduces the bitter taste and increases the soly of caffeine in water, which is the main reason for their combination in food products.[5]Sodium salicylate has an effect on improving lubricating properties of 1-octadecyl-3-nonyl imidazolium bromide wormlike micelles in glycerol/propylene glycol at low temperatures.[6]On the other hand, Sodium salicylate is also a nonsteroidal anti-inflammatory drug can produce the effect on adipocyte browning.[8] and it is a compound that possesses similar antiinflammatory potency as aspirin but lacks aspirin’s inhibitory effect on the activity of isolated cyclooxygenase. Antioxidant properties of salicylates, adenosine release induced by sodium salicylate and aspirin-triggered lipoxin formation are additional mechanisms that may contribute to anti-inflammatory properties of aspirin and/or sodium salicylate.Additionally, Sodium salicylate, serving as a chemical trap for hydroxyl radicals, the most damaging reactive oxygen species, has been shown to ameliorate hypoxia/reoxygenation injury in several tissues.[7]
References
[1]Zhang, Junmei,Wang, Jie.A kind of device and method for efficiently producing sodium salicylate.[P].CN115430380.2022,12,06.
[2]Mitchell JA, et al. Sodium salicylate inhibits cyclo-oxygenase-2 activity independently of transcription factor (nuclear factor kappaB) activation: role of arachidonic acid. Mol Pharmacol. 1997, 51(6):907-12.
[3]Juhyun Oh, et al. Age-associated NF-κB signaling in myofibers alters the satellite cell niche and re-strains muscle stem cell function[J]. Aging (Albany NY). 2016,8(11): 2871–2884.
[4]Nixon M, et al. Salicylate downregulates 11β-HSD1 expression in adipose tissue in obese mice and in humans, mediating insulin sensitization[J]. Diabetes. 2012, 61(4):790-6.
[5]Vrane? M, Borovi? T T, Drid P, et al. Influence of Sodium Salicylate on Self-Aggregation and Caffeine Solubility in Water—A New Hypothesis from Experimental and Computational Data[J]. Pharmaceutics, 2022, 14(11): 2304.
[6]Hu Y, Sun J, Cai Z, et al. Effect of Sodium Salicylate on Improving the Lubricating Properties of [C18imC9] Br Wormlike Micelles in Glycerol/Propylene Glycol at Low Temperatures[J]. Sustainable Chemistry Engineering, 2022, 10(40): 13514-13524.
[7]Amann R, Peskar B A. Anti-inflammatory effects of aspirin and sodium salicylate[J]. European journal of pharmacology, 2002, 447(1): 1-9.
[8]Choi HE, Jeon EJ, Kim DY, et al. Sodium salicylate induces browning of white adipocytes via M2 macrophage polarization by HO-1 upregulation[J]. European Journal of Pharmacology, 2022, 928: 175085.
You may like
Related articles And Qustion
See also
Lastest Price from Sodium salicylate manufacturers

US $0.00/KG2025-10-14
- CAS:
- 54-21-7
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month
US $0.00-0.00/kg2025-04-15
- CAS:
- 54-21-7
- Min. Order:
- 1kg
- Purity:
- 99.0%
- Supply Ability:
- 1tons