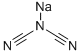
Sodium Dicyanamide: Properties, Applications and Reaction Studies
Sodium dicyanamide is a versatile compound known for its unique properties and applications in various industries. This white crystalline solid is recognized for its high solubility in water and ability to act as a nitrogen source, making it particularly valuable in agricultural formulations. Researchers and industry professionals utilize sodium dicyanamide as a potent fertilizer additive, enhancing the nitrogen content in soil and promoting plant growth. Its effectiveness in stabilizing nitrogen compounds also makes it a key ingredient in the production of slow-release fertilizers, which help reduce nutrient leaching and improve crop yield.

Ignition Delay Reduction with Sodium Addition
A range of ionic liquids (ILs) have been synthesized and modeled to better understand the role of the cation in the ignition of hypergolic ionic liquids. Vogelhuber et al. have shown by density functional theory methods that the addition of sodium cations to an ionic liquid promotes ignition with white fuming nitric acid (WFNA) by lowering energy barriers. To validate this prediction, solid sodium dicyanamide (Na+DCA–) was added at various weight percents to 1-butyl-3-methylimidazolium dicyanamide (BMIM+DCA–). The ignition delay was measured for each mixture with WFNA. Overall, it was found that the sodium dicyanamide lowered the ignition delay by 11 ms at 7 wt %. The calculations done by Vogelhuber et al. appear to be consistent with this observation. The sodium cation may play a role by orienting the anion with the WFNA resulting in the favorable reaction energetics observed.
The BMIM+DCA– was purchased from Io-Li-Tec at greater than 98% purity. The sodium dicyanamide was added at 0, 1, 2, 4, 5, 7, and 10 wt % into the BMIM+DCA–. Gentle shaking of the mixture vials allowed adequate suspension/solution of the solid addition. Over time, the higher weight percents of the sodium dicyanamide mixtures showed some settling of the suspension. Adequate mixing was ensured prior to all testing. Droplet tests are a conventional method for examining ignition delay times of hypergolic fuel/oxidizer combinations. In this study, a syringe pump provided one droplet of the ionic liquid mixture into approximately 1 mL of WFNA (100% Fluka) in a tall 10 mL glass beaker. The ignition delays were recorded by a high speed camera and taken to be the time between first contact of the fuel and oxidizer to the first sign of flame. All data were recorded at 3000 fps. Between 10 and 15 tests were conducted for each combination, and the mean and 95% confidence intervals were calculated. Attenuated total reflectance (ATR) spectra were recorded for the 0–10 wt % sodium dicyanamide in BMIM+DCA– samples as well as the pure sodium dicyanamide. These spectra were used to identify unique infrared frequencies indicating interactions between the Na+ and BMIM+DCA–. The nature of the interactions presented at these frequencies was further analyzed by computational techniques.[1]
A decrease in the average ignition delay times is seen as the weight percent of Na+DCA– is increased. Most notably, there is a 19% and 11 ms reduction in ignition delay between the 100% BMIM+DCA– and the 93 wt % BMIM+DCA– with sodium dicyanamide, showing that the addition of sodium promotes faster ignition kinetics. The average ID for the 10 wt % sample increases from the 7% sample. This may be correlated with the visible settling in the solutions with higher amounts of Na+DCA–, potentially reaching a solubility limit around 7 wt % Na+DCA–. Being contained within a solid particle above this limit, the sodium is less able to interact with the fluid components to affect ignition. Considering the aim for new bipropellants to have an ignition delay less than 10 ms, the addition of an additive like sodium dicyanamide to decrease the ignition delay of an ionic liquid by 11 ms is quite significant. To investigate the structure of the Na+DCA– in BMIM+DCA–, attenuated total reflectance (ATR) infrared spectra were recorded for the 0, 1, 5, 10, and 100 wt % Na+DCA– samples. The results for the 0, 10, and 100 wt % sodium dicyanamide are shown.
A Novel Strategy of In Situ Trimerization of Cyano Groups Between the Ti3C2Tx (MXene) Interlayers
2D MXenes are attractive for energy storage applications because of their high electronic conductivity. However, it is still highly challenging for improving the sluggish sodium (Na)-ion transport kinetics within the MXenes interlayers. Herein, a novel nitrogen-doped Ti3C2Tx MXene was synthesized by introducing the in situ polymeric sodium dicyanamide (Na-dca) to tune the complex terminations and then utilized as intercalation-type pseudocapacitive anode of Na-ion capacitors (NICs). The sodium dicyanamide can intercalate into the interlayers of Ti3C2Tx nanosheets and simultaneously form sodium tricyanomelaminate (Na3TCM) by the catalyst-free trimerization. The as-prepared Ti3C2Tx/Na3TCM exhibits a high N-doping of 5.6 at.% in the form of strong Ti–N bonding and stabilized triazine ring structure. Consequently, coupling Ti3C2Tx/Na3TCM anode with different mass of activated carbon cathodes, the asymmetric MXene//carbon NICs are assembled. It is able to deliver high energy density (97.6 Wh kg−1), high power output (16.5 kW kg−1), and excellent cycling stability (≈ 82.6% capacitance retention after 8000 cycles).[2]
Ti3C2Tx/Na3TCM composite was prepared by a simple hydrothermal for the thermal trimerization of sodium dicyanamide (Na-dca) to sodium tricyanomelaminate (Na3TCM) between Ti3C2Tx interlayers. In a typical synthesis, 200 mg of Na-dca was dissolved in 40 mL of deionized water. After that, 200 mg of Ti3C2Tx was dispersed in the as-obtained sodium dicyanamide solution and stirred at room temperature at 400 rpm for 2 h. Then, the mixture was sealed in the N2 atmosphere and sonicated in an ice bath at 250 W for 1 h to produce a homogeneous solution. Subsequently, the mixed solution was transferred to a Teflon-lined autoclave and the temperature was raised to 180 °C at a heating speed of 2 °C and then maintained for 6 h. The resulting powder was washed several times with deionized water during vacuum filtration and then dried at 60 °C in the vacuum oven.
Theoretical Investigation of the Reactivity of Sodium Dicyanamide with Nitric Acid
There is a need to replace current hydrazine fuels with safer propellants, and dicyanamide (DCA–)-based systems have emerged as promising alternatives because they autoignite when mixed with some oxidizers. Previous studies of the hypergolic reaction mechanism have focused on the reaction between DCA– and the oxidizer HNO3; here, we compare the calculated pathway of DCA– + HNO3 with the reaction coordinate of the ion pair sodium dicyanamide with nitric acid, Na[DCA] + HNO3. Enthalpies and free energies are calculated in the gas phase and in solution using a quantum mechanical continuum solvation model, SMD-GIL. The barriers to the Na[DCA] + HNO3 reaction are dramatically lowered relative to those of the reaction with the bare anion, and an exothermic exit channel to produce NaNO3 and the reactive intermediate HDCA appears. These results suggest that sodium dicyanamide may accelerate the ignition reaction.[3]
In this work, we have presented the calculated reaction coordinate of HNO3 with sodium dicyanamide, with comparisons to the bare anion DCA– and the IL ion pair [MMIM][DCA]. Further, we have found that in the Na[DCA] + HNO3 system there exists a low-lying, net-exothermic exit channel to directly produce the reactive intermediate HDCA. This exit channel becomes even more favorable in the solution phase, where SMD-GIL calculations predict that the reaction of Na[DCA] + HNO3 solvated in [EMIM][DCA] is increasingly negative at each stationary point with no free energy barriers, indicating that the dissociation reaction is spontaneous.
References
[1]Thomas, Anna et al. “Ignition Delay Reduction with Sodium Addition to Imidazolium-Based Dicyanamide Ionic Liquid.” The journal of physical chemistry. A vol. 123,1 (2019): 10-14.
[2]Liu S, Hu F, Shao W, Zhang W, Zhang T, Song C, Yao M, Huang H, Jian X. A Novel Strategy of In Situ Trimerization of Cyano Groups Between the Ti3C2Tx (MXene) Interlayers for High-Energy and High-Power Sodium-Ion Capacitors. Nanomicro Lett. 2020 Jun 25;12(1):135.
[3]Vogelhuber, Kristen M et al. “Theoretical Investigation of the Reactivity of Sodium Dicyanamide with Nitric Acid.” The journal of physical chemistry. A vol. 122,8 (2018): 1954-1959.
See also
Lastest Price from Sodium dicyanamide manufacturers

US $120.00/kg2025-04-15
- CAS:
- 1934-75-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10ton
US $0.00-0.00/kg2025-04-04
- CAS:
- 1934-75-4
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton