Side effects of Ofloxacin
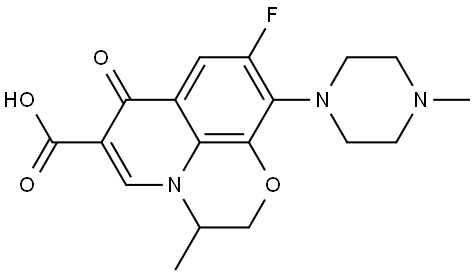
Ofloxacin is a fluoroquinolone antibiotic first synthesized at Daiichi Pharmaceutical Company, Japan, in 1980. It has the chemical formula 9-fluoro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-2,3-dihydro-7Hpyrido-( 1,2,3-de)1,4-benzoxazine-6-carboxylic acid.It is a racemic mixture of S-(–)-isomer DR-3355 and R-(+)-isomer DR-3354. The S-isomer has 8- to 128-fold more antibacterial potency than the R-isomer.

Uses
To take advantage of this enhanced activity, the S-isomer has been developed further as levofloxacin. The antibacterial spectrum of ofloxacin is similar to that of ciprofloxacin, but there are some differences. After initial development in Japan, ofloxacin was licensed to Hoechst for the European market. It was approved by the United States Food and Drug Administration (FDA) in 1990 and was then marketed by Johnson and Johnson for the United States market.
Currently, ofloxacin is available in the USA as "Floxin"(Ortho-McNeil Pharmaceuticals, NJ, USA), in Canada as "Floxin" (Janssen-Ortho, ON, Canada), in Europe as "Tarivid" (Sanofi-Aventis), in Asia as "Tarivid" (Daiichi-Sankyo) and generically worldwide in 200-, 300- and 400-mg tablets, as well as i.v. preparation, ophthalmic drops (3 mg/ ml, 3%) and otic solution (3 mg/ml, 3%).
Drug interactions
Ofloxacin does not impair hepatic metabolism of drugs to any major extent. Gregoire et al. noted that coadministration of ofloxacin with theophylline for 1 day did not change theophylline clearance, but that co-administration for 4 days resulted in a statistically significant decrease (12.1%) in clearance; despite this, there was no increase in clinical adverse reactions. Oral theophylline 200 mg bid for 8 days given with ofloxacin 600 mg orally daily increased ofloxacin Cmax by 9% and AUC by 11% .
Thus, although there is statistically an interaction between ofloxacin and methylxanthines such as theophylline or caffeine, this is not clinically significant and no dosage alteration of these latter compounds is necessary during co-administration.In this regard, ofloxacin has less propensity for interaction with theophylline or caffeine than enoxacin or ciprofloxacin, and appears to be roughly comparable to the other fluoroquinolones lomefloxacin, fleroxacin, levofloxacin, gatifloxacin, and moxifloxacin.
Overall, ofloxacin has clinical activity roughly comparable to that of ciprofloxacin, but ofloxacin offers the potential advantage of being less likely to interact with other concomitantly administered agents such as theophylline, caffeine and fenbufen (see Chapter 93, Ciprofloxacin). Since there have been few head-to-head comparisons, clinicians are left to choose between ofloxacin and ciprofloxacin based on their clinical experience. Against pathogens susceptible to both agents, such as H. influenzae, Gram-negative cocci, and most Enterobacteriaceae, both ofloxacin and ciprofloxacin appear comparable, although ofloxacin is slightly less active than ciprofloxacin against P. aeruginosa. Unlike ciprofloxacin, ofloxacin is effective in genitourinary infections due to C. trachomatis.
Side effects
Ofloxacin has now been used for many years in a large number of patients and is generally considered a safe drug. The overall rate of adverse reactions reported for ofloxacin in clinical trials is 2.4–12.3%, which compares favorably with other fluoroquinolones such as ciprofloxacin. Side effects are similar to those reported with other fluoroquinolones, including gastrointestinal symptoms (nausea 2.6–3.5%, vomiting 3%, diarrhea 1%, abdominal pain 1%), rashes, dizziness (1.2%), headache (1.4%), insomnia (1.8%), and general pruritus or rash (0.5–0.9%); they are usually mild to moderate, and are generally rapidly reversible. The most common toxicity is nausea, with rates lower than with many other fluoroquinolones and comparable to ciprofloxacin. Most adverse events occur within the first 7–10 days of therapy. In only about 0.5–4.0% of patients is cessation of therapy necessary due to adverse reaction, a rate comparable to ciprofloxacin and levofloxacin. Laboratory abnormalities are uncommon, but include reduced neutrophil count (not true neutropenia; 0.98%), hypoglycemia (0.91%), eosinophilia (0.88%), relative lymphocytosis (0.74%), and elevated liver transaminases (0.69%) .
Postmarketing studies in Germany have noted rare reports of hallucinations, psychotic reaction, and shock, which were not noted in earlier clinical trials. Other adverse reactions reported for ofloxacin are described below.
See also
Lastest Price from Ofloxacin manufacturers

US $0.00-0.00/kg2025-09-15
- CAS:
- 82419-36-1
- Min. Order:
- 1kg
- Purity:
- 98.5%-101.5%;USP
- Supply Ability:
- 1000KGS

US $0.00/kg2025-04-25
- CAS:
- 82419-36-1
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg