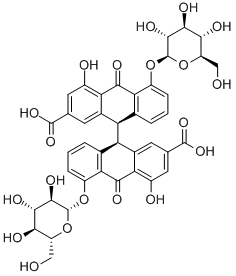
Sennoside A: Physicochemical property, Benefits, and Metabolism
Description
Senna, a quick-acting, reliable stimulating laxative, is generally taken as a tea. Its mechanism of action is primarily via anthracoids (sennoside A and B) or anthraquinone glycosides. Senna is considered appropriate for acute cases and not an herb to be used regularly. Sennosides, a class of natural anthraquinone derivatives, and dimeric glycosides are the main bioactive components from medicinal plants used for traditional herbal laxatives, such as Senna alexandrina Mill. (Senna) and Rheum Officinale Baill (Rhubarb). Among them, sennoside A and B (SA, SB) are the main purgative components, which were first isolated and identified from the leaves of Senna and then attributed to the anthraquinone family by Stoll. Later, two other pharmacologically active sennosides were isolated from the same plant senna, including sennoside C and D (SC, SD). In addition, SA, SB, and SC have also been isolated from Rhubarb[1-2].
Physicochemical property
The SA has some physicochemical properties of LogP: 1.88, molecular formula: C42H38O20, molecular weight: 862.7, melting point: 200–203°C, sparingly soluble in MeOH, insoluble in water, and low bioavailability. Furthermore, SA can be slowly isomerized to its stereoisomer SB with the same molecular formulae and identical substituent (H) located in opposite directions in NaHCO3 solution at 80°C.
Benefits
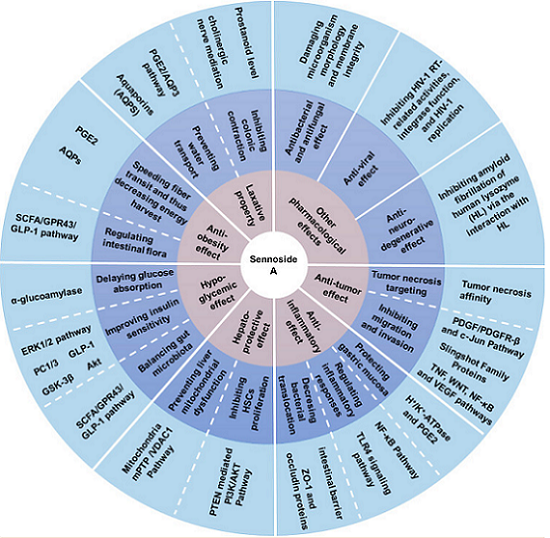
As the most important family member of sennosides, SA is a type of irritant laxative, weight-loss herbal medicine, or dietary supplement that has been routinely used for a long history in China and other Asian countries. SA has been shown to possess other potential pharmacological and therapeutic applications, including anti-obesity, hypoglycemic, hepatoprotective, anti-inflammatory, and anti-cancer effects. Thus, SA has excellent potential to treat various illnesses, such as constipation, obesity, diabetes, fatty liver, and many inflammatory and cancers. However, some other studies have suggested that high-dose and long-term use of SA may induce melanosis coli and subsequent colon cancer.
Metabolism
The possible metabolic patterns of SA, including SA metabolism, absorption, and excretion, illustrated the general scheme from unabsorbed glycosidic into absorbed pharmacologically active aglycon anthrone and excreted metabolites. Sennosides were not hydrolyzed by acid in the stomach nor by ß-glucosidases in the small intestine and, therefore, were unabsorbable by intestinal epithelial cells. After reaching the large intestine, sennosides were transformed to rhein anthrone by ß-glucosidase and reductase of intestinal microflora in two main metabolic pathways.
There are two proposed metabolic pathways of SA. The SA may undergo a hydrolysis reaction to generate sennidin A, then convert it to rhein-anthrone by breaking the C10-10′ bond. After incubation with rat and mouse feces, the rhein anthrone and sennidin A were identified as SA metabolites through intestinal flora. In the second one, SA may undergo hydrolysis to generate rhein-9-anthrone-8-glucoside radical, which is then converted to 8-Glucosyl-rhein-9-anthrone. Finally, the 8-Glucosyl-rhein-9-anthrone generates rhein-anthrone under hydrolase[3].
Then, SA's intestinal metabolite rhein anthrone was absorbed in the colon and underwent enterohepatic recirculation. Studies on urine and fecal excretion showed that after absorption of rhein anthrone, it was oxidized to rhein, which is excreted through urine and feces by combing with glucuronic acid or sulfuric acid. After oral administration of sennosides in the rats, rhein, sennidin, rhein monosulphate, and rhein monoglucuronide were detected in the urine. The sennidin, rhein, and rhein anthrone were detected in feces. In addition, Yin et al. verified that 131I-SA is mainly excreted by the kidney (73.5% excreted by urine and 10.5% excreted by feces). 131I-SA could reach the maximum plasma concentration (Cmax, 163.316 ± 11.180 mBq/L) at 0.083 h, possessing fast blood clearance with an elimination half-life of 6.711 ± 0.564 h.
References
[1] Romm, A. Laurel Lee and C. Hobbs. “CHAPTER 15 – Pregnancy: Third Trimester.” 2010:370-397.
[2] Wen Gao . “Inhibition behavior of Sennoside A and Sennoside C on amyloid fibrillation of human lysozyme and its possible mechanism.” International Journal of Biological Macromolecules 178 (2021): Pages 424-433.
[3] Jiamei Le. “Pharmacology, Toxicology, and Metabolism of Sennoside A, A Medicinal Plant-Derived Natural Compound.” Frontiers in Pharmacology (2021): 714586.
You may like
See also
Lastest Price from Sennoside A manufacturers

US $0.00/KG2025-04-21
- CAS:
- 81-27-6
- Min. Order:
- 1KG
- Purity:
- 95%-99% HPLC
- Supply Ability:
- 1000KG

US $0.00-0.00/kg2025-04-16
- CAS:
- 81-27-6
- Min. Order:
- 20kg
- Purity:
- 99%
- Supply Ability:
- 200000