Sebacic acid—an aliphatic dibasic acid
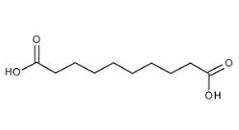
Sebacic acid, also known as 1, 10-decanedioic acid, belongs to aliphatic dibasic acid. Sebacic acid was present in the leaves of flue-cured tobacco, burley tobacco and aromatic tobacco. Sebacic acid was white crystal in flake form at room temperature. Slightly soluble in water, sebacic acid was insoluble in benzene, petroleum ether, carbon tetrachloride. In contrast, sebacic acid was soluble in ethanol and ethyl ether. Irritant to the eyes, respiratory system and skin irritation, sebacic acid oral harmful. However, sebacic acid was low toxic and flammable.
Sebacic acid is widely used in the preparation of sebacic acid esters, such as dibutyl sebacate, dioctyl sebacate, diisooctyl sebacate. These esters can be used as plasticizers for plastics and cold-resistant rubber, as well as for polyamide, polyurethane, alkyd resin, synthetic lubricating oil, lubricating oil additives, spices, coatings, cosmetics, etc. It can also be used as raw material for producing nylon 1010, nylon 910, nylon 810, nylon 610, nylon 9 and high temperature resistant lubricating oil diethylhexyl ester. It is also the raw material for the production of alkyd resins (used as surface coatings, plasticized nitrocellulose coatings, and urea resin varnishes) and polyurethane rubber, cellulose resins, vinyl resins, and synthetic rubber plasticizers, softeners, and solvents.
Sebacic acid was first obtained by retorting of castor oil in present of alkali. Then, a synthetic method was developed. Currently, a new route employing n-alkanes to produce sebacic acid through fermentation was under developing. Another production method of sebacic acid is the electrolytic oxidation method of adipic acid developed by Asahi chemical industry co. LTD. Due to the cost, it was not widely used.
Preparation for sebacic acid:
(1) cracking method of castor oil: castor oil was heated and hydrolyzed under the action of alkali to produce sodium castor oleate soap, and then sulfuric acid was added to produce castor oleic acid. In the presence of the diluent cresol, alkali was added and heated to 260-280℃ for cracking to produce disodium sebacate, octanol and hydrogen. Diluted with water, the disodium salt was heated and neutralized with acid to turn the disodium salt into mono-sodium salt. Then, activated carbon was used for decolorization. The neutral liquid was heated to be boiling and then with the addition of acid sebacic acid mono-sodium salt was acidolyzed into sebacic acid to precipitate. Then, after separation, drying, sebacic acid could be obtained.
(2) fermentation of n-decane: sebacic acid can be obtained by fermentation of n-decane with 19-2 candida lipolytica.
(3) new method employing cyclopentanone as substrate: using palladium-copper or iron as catalyst, cyclopentanone is oxidized by air to cyclopentanone in ethanol, propanol or other alcohols under low temperature and moderate atmospheric pressure at 40-60℃. Then, cyclopentanone is oxidized and dimerized with iron catalyst to form sebacic acid.
References
[1]. 王兰芝,张景涛 氯化铁催化合成癸二酸二丁酯的研究, 化学研究与应用, 2000, 4, 416-418.
[2]. 马建成,夏清,张凤宝,等蓖麻油裂解制癸二酸生产工艺研究进展, 化学工业与工程, 2007, 4, 362-366
You may like
Related articles And Qustion
Lastest Price from Sebacic acid manufacturers

US $80.00/kg2025-08-29
- CAS:
- 111-20-6
- Min. Order:
- 1000kg
- Purity:
- 99.50%
- Supply Ability:
- 50 ton per month

US $3.00/kg2025-04-21
- CAS:
- 111-20-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000
![3680-69-1 4-Chloro-7H-pyrrolo[2,3-d] pyrimidine;preparation; synthesis;intermediate](/NewsImg/2024-05-25/6385226121244282863968209.jpg)


![3680-69-1 4-Chloro-7H-pyrrolo[2,3-d] pyrimidine;preparation; synthesis;intermediate](httpss://www.chemicalbook.com/NewsImg/2019-12-18/2019121895217670.jpg)
