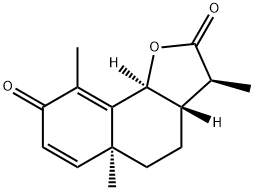
Santonin: Structural Understanding, Microbial Transformation and Bioactivities
General Description
Exploring santonin spans various aspects, from its structural elucidation to microbial transformations and diverse bioactivities. Initially isolated from Artemisia Santonica, santonin's structure was the subject of much debate until Clemo's work in 1929 resolved uncertainties, paving the way for further understanding. Microbial transformations, particularly by fungi like Rhizopus stolonifer and Cunninghamella spp., highlighted the regiospecific reduction of alpha-santonin's carbon-carbon double bond, yielding metabolites with altered properties. These transformations underscore fungi's potential to catalyze complex reactions. Additionally, santonin and its derivatives exhibit diverse bioactivities, including anti-cancer effects. Alpha-santonin derivatives have shown promise against various cancer cell lines, emphasizing the significance of structural modifications in optimizing bioactivity. Further research elucidating mechanisms of action and conducting clinical trials will be crucial in harnessing the therapeutic potential of santonin derivatives. Clemo's structural insights, microbial transformations, and the diverse bioactivities of santonin derivatives collectively highlight their significance in pharmaceutical research and potential for future drug development.

History and structure
Santonin was first isolated by Kahler in 1830 from Artemisia Santonica, a member of a family of plants known for centuries as "wormseed". The name attests to their remarkable efficacy in ridding humans and animals of debilitating roundworms (Nematodes). Early knowledge of its chemistry came almost entirely from the investigations the Italian chemist Cannizzaro and his school carried out between 1870 and 1920. Elemental analysis for carbon and hydrogen (oxygen by difference) established the empirical formula C15H18O3. The difference of 14 between its 18 hydrogen atoms and the 32 hydrogen atoms required by a saturated hydrocarbon C15H32 divided by two implied seven degrees of unsaturation.
The behaviour of santonin on treatment with dilute base or acid, as well as towards reagents regarded as being able to indicate the presence of a carbonyl group, led the Italian researchers to conclude that santonin is a γ-keto lactone. This inference accounted for all three oxygen atoms and three degrees of unsaturation (two carbonyl groups and the lactone ring). Reduction with hydrogen iodide and red phosphorus gave a monobasic acid, santonous acid, which, on distillation with barium hydroxide at 300 ℃, gave a phenol, C12H12O, recognized as 1,4-dimethyl-2- naphthol. Heating at still higher temperatures led to the identification of propene and propionic acid. A three-carbon fragment, two methyl groups, and the C10 naphthalene now accounted for all 15 carbon atoms in santonin. Two fused six-membered rings accounted for two more elements of unsaturation, bringing the total to five. The two remaining seven degrees of unsaturation required by the empirical formula were assigned by default to two carbon-carbon double bonds.
Bioactivities
α-Santonin was recorded in the pharmacopoeia of many countries in the early days. However, in recent years, it has been removed from the pharmacopoeia of some countries due to related side effects such as liver and kidney toxicity, mental disorders, etc. Nowadays, a-santonin is commercially available and has been widely used as a synthon while producing eudesmanolide compounds. Besides anti-roundworm activity, a-santonin possesses diverse bioactivities, including cytotoxicity, antioxidant, anti-inflammation, anti-microbial, P-glycoprotein (P-gp) antagonist, anti-ulcer, anti-viral, anti-protozoal, anti-nematode, antipyretic, reversing behaviour of cocaine-induced planarian, anti-hyperglycemic, analgesic and so on. Interestingly, it was reported that a series of a santonin derivatives presented more and better bioactivities, such as anti-cancer, herbicidal, immunosuppressant, phytotoxic, anti-angiogenic, etc.
Reference
1. Birladeanu L. The stories of santonin and santonic acid. Angew Chem Int Ed Engl. 2003; 42(11): 1202-1208.
2. Ata A, Nachtigall JA. Microbial transformations of alpha-santonin. Z Naturforsch C J Biosci. 2004; 59(3-4): 209-214.
3. Wang J, Su S, Zhang S, et al. Structure-activity relationship and synthetic methodologies of α-santonin derivatives with diverse bioactivities: A mini-review. Eur J Med Chem. 2019; 175: 215-233.
Related articles And Qustion
See also
Lastest Price from Santonin manufacturers
US $15.00/g2022-07-06
- CAS:
- 481-06-1
- Min. Order:
- 1g
- Purity:
- ≥98%
- Supply Ability:
- 1000.00 kgs
US $1.10/g2021-06-19
- CAS:
- 481-06-1
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons Min