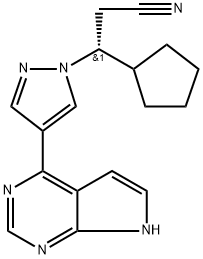
Ruxolitinib: Clinical Applications in Hemophagocytic Lymphohistiocytosis and Adverse Events
General Description
Ruxolitinib is a selective JAK inhibitor that has shown promise as both a salvage and potential front-line therapy for Hemophagocytic Lymphohistiocytosis (HLH). It effectively reduces hyper-inflammatory responses, demonstrating rapid clinical improvements and normalization of inflammatory markers in patients. Despite being associated with various adverse events, including infections and hematological complications, ruxolitinib has a favorable response rate, particularly in refractory cases and among patients with viral triggers. Ongoing studies are crucial to establish optimal dosing, safety profiles, and its role in redefining HLH management, highlighting both its therapeutic potential and necessity for careful monitoring during treatment.

Figure 1. Ruxolitinib
Clinical Applications in Hemophagocytic Lymphohistiocytosis
Introduction to Ruxolitinib and Hemophagocytic Lymphohistiocytosis
Ruxolitinib, a selective Janus kinase (JAK) inhibitor, has emerged as a prominent therapeutic agent in the treatment of Hemophagocytic Lymphohistiocytosis (HLH), particularly given its ability to dampen hyper-inflammatory responses associated with this condition. Initially, studies by Das et al. and Maschalidi et al. demonstrated the efficacy of ruxolitinib in mouse models of HLH, which prompted further investigation in human patients. To date, there have been 18 independent studies detailing the treatment of 115 unique patients with HLH using ruxolitinib, primarily in adults with secondary HLH. This salvage therapy has shown significant clinical improvements within as little as 24 to 48 hours, alleviating symptoms such as fever and respiratory distress. Furthermore, laboratory markers indicative of inflammation, including ferritin and soluble IL-2 receptor, often normalize within one month, highlighting ruxolitinib's rapid and effective treatment profile. 1
Efficacy as Salvage Therapy
Ruxolitinib has been consistently reported to induce favorable responses in patients with refractory HLH when used as part of a salvage regimen. For instance, the profound impact of ruxolitinib was illustrated in a case involving an 11-year-old boy who exhibit severe HLH unresponsive to conventional therapies. Following the introduction of ruxolitinib, the patient rapidly improved, featuring resolved fever and enhanced organ function within 24 hours. Additional studies underscore ruxolitinib's potential, with a notable report describing a 74% response rate among 34 patients treated with ruxolitinib and corticosteroids for secondary HLH. Furthermore, relapses are a concern, especially in patients with EBV-related HLH, necessitating ongoing investigation into ruxolitinib's long-term efficacy. Overall, these findings suggest that ruxolitinib not only effectively mitigates inflammation but also serves as a critical option in cases resistant to traditional HLH therapies, reinforcing its place in salvage protocols. 1
Ruxolitinib as a Potential Front-Line Therapy
While ruxolitinib has largely been applied as a salvage therapy, early studies suggest its viability as a front-line treatment for HLH. Research indicates that ruxolitinib can be beneficial when initiated alongside other therapies, as exhibited by cases of adults with secondary HLH triggered by infections. Reports showcase the improvement of multi-organ failure and cytopenias, reinforcing ruxolitinib’s role in stabilizing critically ill patients. A recent open-label study enrolling multiple adults also documented rapid improvements in hemoglobin levels and ferritin within the first two weeks of ruxolitinib treatment. In pediatric populations, ruxolitinib displays a promising response rate, especially among patients with viral triggers. However, further research is imperative to establish optimal dosing strategies and timing for ruxolitinib in HLH management. The evolving data underscore ruxolitinib's potential to redefine first-line treatment approaches in HLH while ensuring comprehensive safety evaluations in diverse patient populations. 1
Adverse Events
Overview of Adverse Events
Ruxolitinib, a selective inhibitor of JAK1 and JAK2, is commonly associated with various adverse events (AEs), particularly non-hematologic issues such as peripheral edema and diarrhea. Studies indicate that patients undergoing prolonged treatment with ruxolitinib experience fewer AEs compared to those who are treated for shorter durations. Infections represent another significant concern, with ruxolitinib-treated patients frequently reporting occurrences of sepsis, viral infections, pneumonia, and urinary tract infections. The pharmacological action of ruxolitinib inhibits hematopoiesis, leading to hematological complications like anemia and thrombocytopenia. The COMFORT-II trial reported that 55% of participants faced serious AEs, warranting close monitoring. Notably, 25% of patients discontinued ruxolitinib treatment due to AEs such as anemia and thrombocytopenia, highlighting the need for careful management through dose adjustments and supportive therapies like corticosteroids and blood transfusions. 2
Renal Function and Safety Considerations
Renal issues have also been documented in patients receiving ruxolitinib, with instances of renal failure emerging during therapy. While some other tyrosine kinase inhibitors (TKIs) similarly pose risks for renal complications, the precise mechanisms remain unclear. It is vital to monitor kidney function rigorously during ruxolitinib treatment since compromised renal function can impact the clearance of the drug's active metabolites, necessitating potential dosage modifications. Interestingly, ruxolitinib does not seem to influence the QTc interval based on studies evaluating its effects compared to known QTc prolonging agents. Furthermore, although concerns arose regarding long-term cancer risks associated with other JAK inhibitors, such as tofacitinib, current data do not suggest that ruxolitinib is linked to increased secondary cancer incidence. This emphasizes the importance of continuous evaluation of safety profiles for ruxolitinib, particularly over prolonged treatment durations. 2
References:
[1] CAMILLE KEENAN S A Kim E Nichols. Use of the JAK Inhibitor Ruxolitinib in the Treatment of Hemophagocytic Lymphohistiocytosis.[J]. ACS Applied Materials & Interfaces, 2021. DOI:10.3389/fimmu.2021.614704.[2] T Y J APPELDOORN. Pharmacokinetics and Pharmacodynamics of Ruxolitinib: A Review.[J]. Clinical Pharmacokinetics, 2023, 62 4. DOI:10.1007/s40262-023-01225-7.
Lastest Price from Ruxolitinib manufacturers

US $0.00-0.00/KG2025-07-01
- CAS:
- 941678-49-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1T

US $5.00/kg2025-04-21
- CAS:
- 941678-49-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000