Relationship between Galactose and Lactose
Description
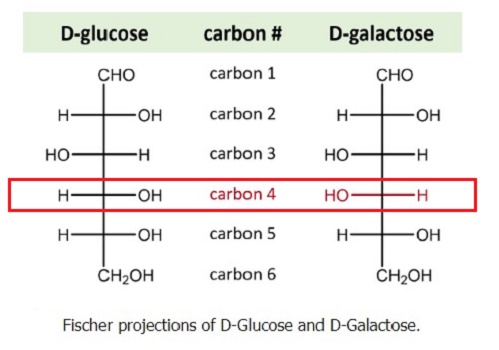
Galactose is an optical isomer of glucose. An aldohexose that occurs naturally in the D-form in lactose, cerebrosides, gangliosides, and mucoproteins. Deficiency of galactosyl-1-phosphate uridyltransferase (Galactose-1-phosphate uridyl-transferase deficiency disease) causes an error in galactose metabolism called galactosemia, resulting in elevations of galactose in the blood. Galactose (Gal) (also called brain sugar) is a type of sugar found in dairy products, in sugar beets and other gums and mucilages. It is also synthesized by the body, where it forms part of glycolipids and glycoproteins in several tissues. It is considered a nutritive sweetener because it has food energy. Galactose is less sweet than glucose and not very water-soluble.
Galactose is a monosaccharide constituent, together with glucose, of the disaccharide lactose. The hydrolysis of lactose to glucose and galactose is catalyzed by the enzyme beta-galactosidase, a lactase. In the human body, glucose is changed into galactose in order to enable the mammary glands to secrete lactose. Galactan is a polymer of the sugar galactose. It is found in hemicellulose and can be converted to galactose by hydrolysis.
Structure and isomerism
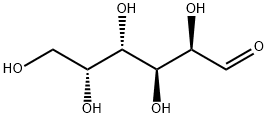
Galactose exists in both open-chain and cyclic form. The open-chain form has a carbonyl at the end of the chain.
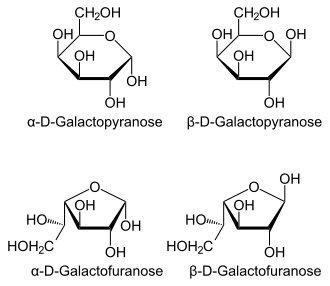
Four isomers are cyclic, two of them with a pyranose (six-membered) ring, two with a furanose (five-membered) ring. Galactofuranose occurs in bacteria, fungi and protozoa, and is recognized by a putative chordate immune lectin intelectin through its exocyclic 1,2-diol. In the cyclic form there are two anomers, named alpha and beta, since the transition from the open-chain form to the cyclic form involves the creation of a new stereocenter at the site of the open-chain carbonyl. In the beta form, the alcohol group is in the equatorial position, whereas in the alpha form, the alcohol group is in the axial position.
Relationship to lactose
Galactose is a monosaccharide. When combined with glucose (monosaccharide), through a condensation reaction, the result is the disaccharide lactose. The hydrolysis of lactose to glucose and galactose is catalyzed by the enzymes lactase and β-galactosidase. The latter is produced by the lac operon in Escherichia coli.
In nature, lactose is found primarily in milk and milk products. Consequently, various food products made with dairy-derived ingredients can contain lactose. Galactose metabolism, which converts galactose into glucose, is carried out by the three principal enzymes in a mechanism known as the Leloir pathway. The enzymes are listed in the order of the metabolic pathway: galactokinase (GALK), galactose-1-phosphate uridyltransferase (GALT), and UDP-galactose-4’-epimerase (GALE).
In human lactation, glucose is changed into galactose via hexoneogenesis to enable the mammary glands to secrete lactose. However, most lactose in breast milk is synthesized from galactose taken up from the blood, and only 35±6% is made from galactose from de novo synthesis. Glycerol also contributes some to the mammary galactose production.
You may like
Related articles And Qustion
See also
Lastest Price from D-Galactose manufacturers

US $1.00/kg2025-04-21
- CAS:
- 59-23-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $999.00-666.00/ton2025-04-21
- CAS:
- 59-23-4
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 5000