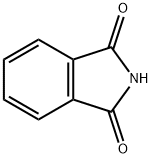
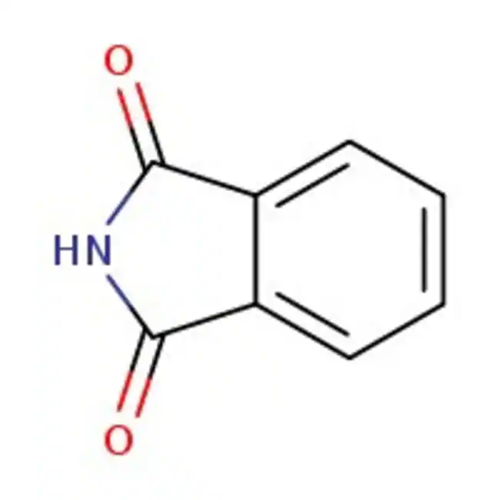
Reaction of Phthalimide: Deprotection and Amino Groups
What is Phthalimide?
Phthalimide is an organic intermediate compound whose primary use is the formation of aliphatic amines. Phthalimide is a chemical precursor for the chemical synthesis of peptides and is also used in the synthesis of o-aminobenzoic acid for the dye industry. It is used as a masking source of ammonia in pharmaceutical manufacturing for the synthesis of various nitrogen-containing compounds. It is more beneficial than free ammonia. It is also used in the preparation of tumour necrosis inhibitors.
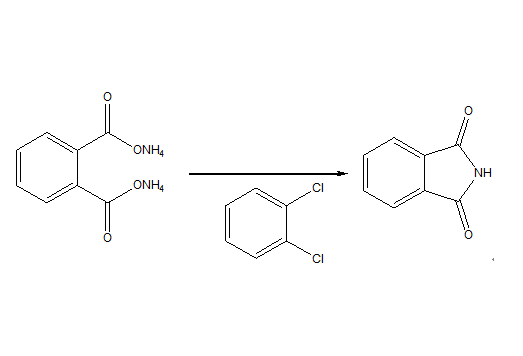
Reaction of Phthalimide
The most important synthetic method for phthalimides is the dehydration condensation of phthalic anhydride with primary amines (if amines are available) at elevated temperatures. When amines are not readily available, direct N-alkylation of phthalimide with alcohols under Mitsunobu conditions and direct N-alkylation of potassium phthalimide with alkyl halides (Gabriel Synthesis) are commonly used alternatives to obtain Phth-protected amines.
Deprotection
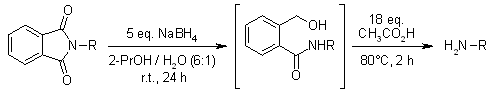
Phthalimides are converted into primary amines by an efficient two-stage single-bottle operation with NaBH4/2-propanol followed by acetic acid. α-Amino acids of phthalimides can be deprotected smoothly and without measurable loss of optical activity.
Suzuki-Miyaura cross-couplings enabled one-time aminomethylation of aryl halides, triflate, methanesulfonate and p-toluenesulfonate by coupling with sodium phthalimidomethyl trifluoroborate followed by deamination with ethylenediamine.
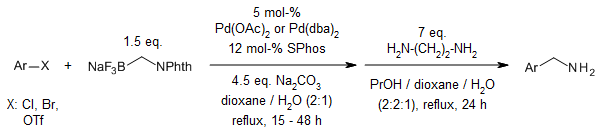
N-acyl- and N-alkoxycarbonylaminophthalimides are efficiently used as acid partners in the Mitsunobu reaction. By means of this reaction they can be alkylated with either a n-, sec- or benzyl group. The 1,1-substituted hydrazines can be efficiently prepared by the final step of dephthaloylation.
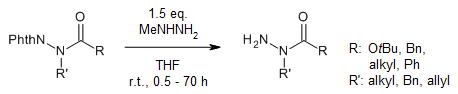
Protection of Amino Groups
The imine was prepared from the corresponding anhydride by a Lewis acid catalysed and solvent-free procedure under microwave irradiation using TaCl5 silica gel as a Lewis acid.
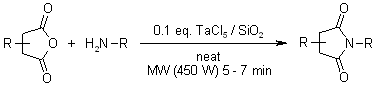
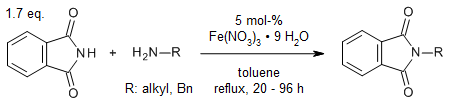
Several primary, secondary and tertiary amines were efficiently transamidated with aliphatic and aromatic amines (primary and secondary amines) in the presence of different hydrated iron(III) salts at 5 mol % concentrations. The method was also applied to urea and phthalimide, in order to demonstrate its versatility and wide substrate range. A plausible mechanism explains the key role of water.
Related articles And Qustion
See also
Lastest Price from Phthalimide manufacturers

US $10.00/KG2025-04-21
- CAS:
- 85-41-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons

US $25.00-9.00/kg2025-03-07
- CAS:
- 85-41-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20 tons