Pterostilbene: pharmacokinetics and clinical applications
General Description
Pterostilbene is a polyphenolic compound that undergoes rapid and extensive conjugation in the intestinal tract, limiting its absorption into the bloodstream. It undergoes phase II metabolism via methylation, glucuronidation, and sulfation. Intravenous administration bypasses these limitations. In animal studies, its half-life was 77.9 minutes. Pterostilbene has potential dermatological applications for skin disorders due to its anti-inflammatory, immunomodulatory, and antioxidant properties. It can inhibit fungal infections and protect against UVB-induced carcinogenesis. In cancer prevention, it inhibits preneoplastic lesions, suppresses inflammation and tumor development, inhibits invasion and metastasis, induces apoptosis and cell cycle arrest, and regulates gene expression. Overall, Pterostilbene shows promise in various clinical applications.

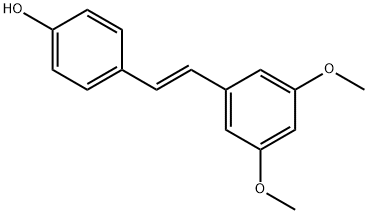
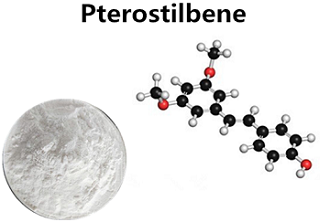
Figure 1. Pterostilbene
Pharmacokinetics
Pterostilbene is a polyphenolic compound that undergoes rapid and extensive conjugation in the human and rodent intestinal tract. This means that it is absorbed with very little of the free compound gaining access to the bloodstream. Most polyphenols are not subjected to phase I metabolism in the liver because the polyphenolic structures make them partially unfavorable substrates for the cytochrome P450 enzymes. However, polyphenols can directly undergo phase II metabolism, predominantly methylation, glucuronidation, and sulfation. After oral administration, polyphenol aglycones undergo extensive phase II metabolism via UDP-glucuronyl transferase isoforms that markedly decrease the amount of unconjugated/natural compounds reaching the systemic blood circulation. Hydrophilic polyphenol conjugates need carriers to cross the enterocyte membrane on the luminal and serosal sides. However, these limitations can be circumvented by intravenous administration. In animal studies, the highest concentration of Pterostilbene in plasma decreased rapidly after intravenous administration, and its half-life was found to be 77.9 minutes. Following oral administration, its absolute oral bioavailability was 12.5%. Pterostilbene concentrations were markedly greater than concentrations of its natural occurring analog, Resveratrol. Multiple enzymemediated methylations can increase the bioavailability of polyphenols, but other studies indicate a marked decrease in their anticancer benefits. In vivo biological activity of equimolar doses of Pterostilbene was greater than that of Resveratrol. Nevertheless, most available data indicate that natural polyphenols (including Pterostilbene) are biologically much more active than their in vivo generated metabolites. 1
Clinical applications
Dermatological applications
Pterostilbene is a type of polyphenol that possesses anti-inflammatory, immunomodulatory, and antioxidant properties. It has potential dermatological applications for a variety of skin disorders, including skin cancer, inflammatory dermatoses, mycoses, and photoprotection. For inflammatory dermatoses, Pterostilbene has been suggested as a possible gold standard for skin anti-inflammatory agents. Although only a few observations have been reported on its effect in specific dermatoses, such as psoriasis, atopic dermatitis, and contact/allergic dermatitis, primary observations using standard models and chemically induced skin alterations in mice show promising results. In the case of mycoses, Pterostilbene has been observed to inhibit fungi caused by dermatophyte like Trichophyton rubrum and Candida albicans by binding chitin in the cell wall and disrupting cell wall integrity. This mechanism of inhibition differs from currently available antifungal drugs, making it a potential alternative for drug-resistant mycoses. In terms of photoprotection, Pterostilbene has been found to have a protective effect against chronic UVB-induced carcinogenesis in SKH-1 hairless mice. Despite having minimal sun protection factor (SPF), it fully protects against skin carcinogenesis, possibly involving a biological mechanism. In this model, Resveratrol was unable to exert full protection, indicating that Pterostilbene could be a more effective agent for photoprotection. Further studies are being conducted to elucidate the molecular targets and mechanisms of action of Pterostilbene in dermatological applications. Overall, Pterostilbene represents a promising group of compounds for skin disorders, and its potential therapeutic uses continue to be explored. 2
Cancer prevention
Pterostilbene, a natural polyphenol found in berries, has shown promising applications in cancer prevention. Studies have demonstrated its ability to inhibit carcinogen-induced preneoplastic lesions, particularly in the skin and colon. In mouse models, Pterostilbene was found to significantly inhibit the formation of preneoplastic lesions in the skin, similar to the effects of Resveratrol (Resv). It also decreased the formation of aberrant crypt foci and reduced the levels of proinflammatory cytokines in the colon. These effects were attributed to the suppression of multiple signal transduction pathways involved in inflammation and tumor development. Pterostilbene exhibited potent activity in inhibiting the activation of NF-kB, AP-1, COX-2, and iNOS, which are important factors in promoting cancer progression. It also demonstrated the ability to suppress invasion, migration, and metastasis of cancer cells, as well as inhibit the expression of key signaling molecules such as VEGF, EGF, and EGFR. Furthermore, Pterostilbene induced apoptosis and cell cycle arrest in breast cancer cells, inhibited lung and prostate cancer cell growth, and showed chemopreventive effects on lung carcinogenesis in mice. It also displayed inhibitory effects on leptin-stimulated breast cancer cell proliferation and JAK/STAT3 signaling. In addition to its direct effects on cancer cells, Pterostilbene was found to regulate the RNA interference pathway by promoting the expression of tumor-suppressive microRNAs. This suggests that pterostilbene may contribute to the prevention and treatment of certain cancers by regulating gene expression. 3
Reference
1. Asensi M, Ortega A, Mena S, et al. Natural polyphenols in cancer therapy. Crit Rev Clin Lab Sci 2011;48:197–216.
2. Chen YY, Lee YH, Wang BJ, Chen RJ, Wang YJ. Skin damage induced by zinc oxide nanoparticles combined with UVB is mediated by activating cell pyroptosis via the NLRP3 inflammasome-autophagy-exosomal pathway. Part Fibre Toxicol. 2022 Jan 5;19(1):2.
3. Estrela JM, Ortega A, Mena S, Rodriguez ML, Asensi M. Pterostilbene: Biomedical applications. Crit Rev Clin Lab Sci. 2013 May-Jun;50(3):65-78.
Related articles And Qustion
See also
Lastest Price from Pterostilbene manufacturers

US $0.00-0.00/kg2025-08-20
- CAS:
- 537-42-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00/Kg/Bag2025-04-21
- CAS:
- 537-42-8
- Min. Order:
- 1KG
- Purity:
- 99%min HPLC
- Supply Ability:
- 100kgs