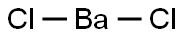Property, preparation and application of barium chloride
General description
Any of a variety of substances that contain barium. Most are whitish colored crystalline solids. They tend to be soluble in water and denser than water. They may be toxic by inhalation or possibly skin absorption [1]. They are often used to make other chemicals. Barium chloride is one of the most important soluble barium salts, which is also known as "salting barium". The formula is BaCl2, and it has colorless monoclinic and colorless cubic two crystals, monoclinic crystal turns into cubic crystal at 962 °C [2]. At room temperature, it is white lustrous monoclinic crystal, odorless, bitter and salty. It is soluble in water, insoluble in acetone, ethanol and ether, slightly soluble in acid, sulfuric acid. Crystallization from aqueous solution of barium chloride often contains two crystal water. When heated to 113 °C, it loses crystal water and becomes anhydrous barium chloride, it is white powder [3].
Chemical properties and physical properties
Barium chloride,BaCI2, is a colorless toxic salt with a melting point of 963 °C. It is soluble in water. Barium chloride is used in metal surface treatment and as a rat poison. Barium chloride has the formula, BaCl2 and is an ionic chemical compound. It is one of the most important water-soluble salts of barium-containing compounds. Like other barium salts, it is toxic and imparts a yellow-green coloration to a flame [4]. It is also hygroscopic. Barium chloride was the by-product of the discovery of radium by Madame Curie. When refining radium, the final separation resulted in barium chloride and radium chloride. BaCl2 crystallizes in both the cubic “fluorite” and “lead chloride” crystal structures, both of which accommodate the preference of the large Ba2+ ion for coordination numbers greater than six [5].
Preparation
Barium chloride can be used for identification and separation of SO42- ions, purify the brine water, mainly used for the manufacture of barium salts and pigments, it can also be used as hard water softener, wool and leather industry mordant, pesticides for controlling plant pests [6]. Preparation of industrial barium chloride is mainly used barite as material which containing high components of barium sulfate barite, coal and calcium chloride is mixed, and calcined to get barium chloride, reaction is as follows:
BaSO4 + 4C + CaCl2 → BaCl2 + CaS + 4CO ↑
Besides, barium chloride dihydrate is heated to above 150 °C by dehydration to obtain anhydrous barium chloride products. Its reaction is in the following [7]:
BaCl2 • 2H2O [△] → BaCl2 + 2H2O
Barium chloride can be prepared from barium hydroxide or barium carbonate, the latter being found naturally as the mineral “Witherite”. These basic salts react to give hydrated barium chloride. On an industrial scale, it is prepared via a two-step process from the mineral “Baryte” [8]:
BaSO4+4C→BaS+4CO (gas)
This first step requires high temperatures. The second step requires fusion of the reactants:
BaS+ CaCl2→BaCl2+CaS
The BaCl2 is then be leached out from the mixture with water. From water solutions of barium chloride, the dihydrate can then be crystallized as white crystals, BaCl2·2H2O, which are colorless, translucent rhomboidal tablets or lamellae. The dihydrate is stable in the air at room temperature, but loses one-half of its water above 55°C(131F), and becomes anhydrous at 121°C (250 F) [9].
Application
Barium chloride (BaCl2) is mainly used for heat treatment of metals, barium salt manufacturing, electronic instruments, and used as water softener. It can be used as dehydrating agent and analysis reagents, it is also used for machining heat treatment calibration instruments and devices, evaluation methodology, quality assurance/quality control. Moreover, barium chloride is used in the manufacture of paint pigments and dyeing textiles and as an additive in oils [10]. It is also used as a water softener. Barium chloride is used in manufacture of pigments, fire works, other barium salts, fireworks , hardening of steel, heat treatment salts, purification of brine solution in caustic chlorine plants, lubrication oil additive, textile dye, pigments, white leather, aluminum refining, boiling water treatment and porcelain enamels for sheet steel. It is also used to remove sulfate ion in some electrolytic plants, caustic soda, magnesium metal or sodium metal. It is also used as a component in a flux used to prevent oxidation of molten magnesium [11].
Industrial uses
Barium chloride (BaCl2·2H2O) is a colorless, white powder highly soluble in water (25% at 10 °C). It is quite a toxic reagent. Barium chloride is used during borite flotation as an activator. Barium chloride also has a depressing effect on fluorite and cassiterite [12].
Hazardous characteristics
Barium chloride is noncombustible. It is highly toxic. When contacts boron trifluoride, violent reaction can occur. Swallowed or inhaled can cause poisoning, it is mainly through the respiratory tract and digestive tract to invade the human body, it will cause drooling and burning esophagus, stomach pain, cramps, nausea, vomiting, diarrhea, high blood pressure, no law firm pulse, cramps, a lot of cold sweat, weak muscle strength, gait, vision and speech problems, difficulty breathing, dizziness, tinnitus, consciousness usually clear [13]. In severe cases, it can cause sudden death. Barium ions can cause muscle stimulant, then gradually transforms into paralysis. Rat oral LD50150 mg/kg, mouse peritoneal LD5054 mg/kg, rats are intravenously LD5020 mg/kg, orally in dog LD5090 mg/kg [14].
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated [15].
Safety Profile
A poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Inhalation absorption of barium chloride equals 60-80%; oral absorption equals 10-30%. Experimental reproductive effects. Mutation data reported. See also BARIUM COMPOUNDS (soluble). When heated to decomposition it emits toxic fumes of Cl- [16].
Health hazards
Acute poisoning after oral administration of barium chloride shows nausea, vomiting, abdominal pain, abdominal cramps, slow pulse, progressive muscle paralysis, arrhythmia, and significantly decreased blood spirit. Death can result from arrhythmia and paralysis of respiratory muscles. Inhalation of smoke containing barium chloride may cause poisoning, but gastrointestinal symptoms are not apparent. Skin burns caused by contact with high-temperature barium chloride solution can be simultaneously absorbed and poisoned [17]. Even more, the long-term contact with barium chloride for workers, they can have weakness, shortness of breath, salivation, oral mucosa swelling erosion, rhinitis, conjunctivitis, diarrhea, tachycardia, high blood pressure, hair loss, etc.
Emergency treatment
Barium chloride is used for closed operation and local exhaust. Operators must be specially trained and strictly abide by the operating procedures. It is recommended that the operator wear a self-priming filter dust mask, halberd chemical safety protection eye wrap, and wear rubber gloves. Operators should also avoid barium chloride dust and contact with oxidizing agents and acids. When transporting barium chloride, workers should wear the male package until unloading to prevent damage to the package and container. Emergency handling equipment needs to be equipped. Because empty containers can be hazardous [18].
Storage condition
Barium chloride should be stored in a cool and ventilated warehouse, away from fire and heat sources. The package is specially sealed. It should be stored separately from oxidants, acids and edible chemicals, and must not be mixed. The storage area shall be equipped with suitable materials to accommodate leaks [19]. The "five pairs" management system for extremely toxic substances should be strictly implemented.
References
[1] E.B. Brackett, T.E. Brackett, R.L. Sass, The crystal structures of barium chloride, barium bromide, and barium iodide, The Journal of Physical Chemistry 67(10) (1963) 2132-2135.
[2] G. Jones, M. Dole, The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride, Journal of the American Chemical Society 51(10) (1929) 2950-2964.
[3] W.H. Hendershot, M. Duquette, A simple barium chloride method for determining cation exchange capacity and exchangeable cations, Soil Science Society of America Journal 50(3) (1986) 605-608.
[4] V. Vitagliano, P.A. Lyons, Diffusion coefficients for aqueous solutions of sodium chloride and barium chloride, Journal of the American Chemical Society 78(8) (1956) 1549-1552.
[5] F. Edwards, R. Howe, J. Enderby, D. Page, The structure of molten barium chloride, Journal of Physics C: Solid State Physics 11(6) (1978) 1053.
[6] R. Bhajantri, V. Ravindrachary, A. Harisha, C. Ranganathaiah, G. Kumaraswamy, Effect of barium chloride doping on PVA microstructure: positron annihilation study, Applied Physics A 87(4) (2007) 797-805.
[7] R.T. Thimma, S. Tammishetti, Barium chloride crosslinked carboxymethyl guar gum beads for gastrointestinal drug delivery, Journal of applied polymer science 82(12) (2001) 3084-3090.
[8] S. Ananda, Z. Shaohua, L. Liang, Fatal barium chloride poisoning: four cases report and literature review, The American Journal of Forensic Medicine and Pathology 34(2) (2013) 115-118.
[9] K.R. Patil, A.D. Tripathi, G. Pathak, S.S. Katti, Thermodynamic properties of aqueous electrolyte solutions. 2. Vapor pressure of aqueous solutions of sodium bromide, sodium iodide, potassium chloride, potassium bromide, potassium iodide, rubidium chloride, cesium chloride, cesium bromide, cesium iodide, magnesium chloride, calcium chloride, calcium bromide, calcium iodide, strontium chloride, strontium bromide, strontium iodide, barium chloride, and barium bromide, Journal of chemical and engineering data 36(2) (1991) 225-230.
[10] R. Robinson, V. Bower, Thermodynamics of the ternary system: water-sodium chloride-barium chloride at 25° C, Journal of Research of the National Bureau of Standards. Section A, Physics and Chemistry 69(1) (1965) 19.
[11] A.B. Morton, C.E. Norton, N.L. Jacobsen, C.A. Fernando, D. Cornelison, S.S. Segal, Barium chloride injures myofibers through calcium-induced proteolysis with fragmentation of motor nerves and microvessels, Skeletal muscle 9(1) (2019) 1-10.
[12] S.F. Wetherill, M.J. Guarino, R.W. Cox, Acute renal failure associated with barium chloride poisoning, Annals of Internal Medicine 95(2) (1981) 187-188.
[13] V. Sokolov, V. Plotnichenko, V. Koltashev, Structure of barium chloride-oxide tellurite glasses, Journal of non-crystalline solids 355(31-33) (2009) 1574-1584.
[14] S. Shanmugan, N. Saravanan, V. Chithambaram, B. Deepanraj, G. Palani, Investigation on single crystal by tartaric acid–barium chloride: growth and characterization of novel NLO materials, Bulletin of Materials Science 43(1) (2020) 1-8.
[15] M.T. Tierney, A. Sacco, Inducing and evaluating skeletal muscle injury by notexin and barium chloride, Skeletal muscle regeneration in the mouse, Springer2016, pp. 53-60.
[16] D. Dietz, M. Elwell, W. Davis Jr, E. Meirhenry, Subchronic toxicity of barium chloride dihydrate administe
Related articles And Qustion
See also
Lastest Price from Barium chloride manufacturers

US $10.00/KG2025-04-21
- CAS:
- 10361-37-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $100.00/KG2024-10-11
- CAS:
- 10361-37-2
- Min. Order:
- 10KG
- Purity:
- 99%
- Supply Ability:
- 10000kg