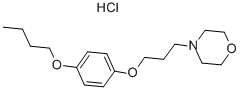
Pramoxine hydrochloride: Application & Preparation
Pramocaine (INN and BAN, also known as pramoxine or pramoxine HCI) is a topical anesthetic discovered at Abbott Laboratories in 1953 and used as an antipruritic. Chemically, it is p-n butoxyphenyl gammamorpholinopropyl ether hydrochloride. During research and development, pramoxine hydrochloride stood out among a series of alkoxy aryl alkamine ethers as an especially good topical local anesthetic agent[1]. Pharmacologic study revealed it to be potent and of low acute and subacute toxicity, well tolerated by most mucous membranes and of a low sensitizing index in humans. Like other local anesthetics, paramoxine decreases the permeability of neuronal membranes to sodium ions, blocking both initiation and conduction of nerve impulses. Depolarization and repolarization of excitable neural membranes is thus inhibited, leading to numbness[2].The popular itch creams Gold Bond and some forms of Calamine Lotion use pramocaine hydrochloride to numb sensitive skin, as does the pain relief variant of Neosporin and some formulations of Sarna. The hydrochloride salt form of pramocaine is water-soluble[3].
Pramoxine hydrochloride can be prepared according to the reported literature[4].
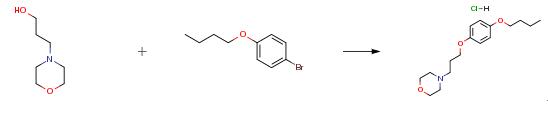
A 500 ml reaction flask was added with 23.2 g (0.1 mol) of the intermediate. 3-morpholine-1-propanol 17.42g (0.12 mol) and 115 g of tetrahydrofuran, with stirring turned on, slowly add 60 percent sodium hydride 4.8g (0.12 mol), temperature below 20°C, about 1~2 min after addition, The reaction was heated to reflux and monitored by TLC. After about 5 hours, the reaction was completed. The solution was concentrated under reduced pressure. 60 ml of water was added and the pH was adjusted to 1 to 2 with concentrated hydrochloric acid. Ethyl acetate (100 ml) was washed twice. The aqueous layer was adjusted to pH 11-12 with 40 percent sodium hydroxide, and the mixture was extracted twice with 120 ml of ethyl acetate. Combine the organic layers, dry an appropriate amount of anhydrous sodium sulfate, decolorize with 2 g of charcoal, concentrate at 40°C without concentration, and dissolve with 80ml of methanol. Concentrated hydrochloric acid to adjust the pH 1~2, vacuum concentration to no more, add acetone 40ml dissolved, stirring crystallization, continue to cool to -10 ° C, stirring crystallization about 6 ~ 8 hours. The mixture was filtered with suction, washed with cold acetone, and dried at 45-50° C. for 6-8 hours to obtain 27.25 g of a white crystalline solid with a yield of 82.61 percent, a purity of 99.926 percent, and a maximum single impurity of 0.046 percent.
References
1.Hanretty KP, Davidson SE, Cordiner JW. Clinical evaluation of a topical anaesthetic preparation (pramoxine hydrochloride and hydrocortisone) in post-episiotomy pain relief[J]. The British journal of clinical practice. 1984,38(11-12):421-2.
2.Gary G, Charles Z. An evaluation of the moisturizing and anti-itch effects of a lactic acid and pramoxine hydrochloride cream[J]. Cutis, 2004, 73(2):135-9.
3.Schmidt JL, Blockus LE, Richards RK. The pharmacology of pramoxine hydrochloride: a new topical local anesthetic[J]. Current Researches in Anesthesia and Analgesia, 1953,32(6:1):418-425.
4.Shandong Cheng Hui Shuang Da Pharmaceutical Co., Ltd. Wang S, Yang Y, Sun B Hu J, Li Y, A method for preparing [...] hydrochloride (by machine translation). CN107892677[P], 2018, A, Paragraph 0019; 002
You may like
Related articles And Qustion
See also
Lastest Price from Pramoxine hydrochloride manufacturers

US $10.00/KG2025-06-27
- CAS:
- 637-58-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 637-58-1
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 900kg