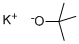Potassium tert-butoxide: physicochemical property and Application
Physicochemical Property
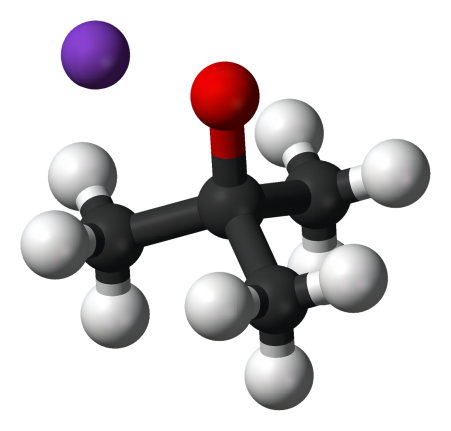
Potassium tert-butoxide is also known as Potassium 2-methylpropan-2-olate or t-BuOK.
Molecular weight: 112.21; Molecular formula: KOC(CH3)3.
Appearance: white to light yellow powder to crystal
Density: 0.902 g/mL at 25 °C
Melting point: 240 °C
Hygroscopic.
Soluble in ether.
It should be stored and handled under inert atmosphere. It is a strong non-nucleophilic base. Its conjugate acid has a pKa of about 17. Similar bases include tert-butanol sodium (t-BuONa) and tert-butanol lithium (t-BuOLi).
Potassium tert-butoxide reagent solutions can be prepared by reacting anhydrous ethanol with potassium in a nitrogen atmosphere.
Application of Potassium tert-butoxide
Potassium tert-butoxide solution(t-BuOK) can be used:
As a catalyst for the interesterification of rapeseed oil with methyl acetate.
To promote Sommelet–Hauser rearrangements of N-benzylic amino acid-derived ammonium ylides under mild conditions.
As a metal-organic precursor in the synthesis of KNbO3 ferroelectric films by the chemical vapor deposition method.
To prepare 3-potassiooxamethylpyridine catalyst.
As a strong alkoxide base reagent.
Potassium tert‐Butoxide Mediated Synthesis of Phenanthridinone.
As a catalyst for the synthesis of γ-butenolide
References:
[1] GUANGFEN DU. Diastereoselective Synthesis of γ-Butenolides Catalyzed by Potassium tert-Butoxide[J]. Synthetic Communications, 2012. DOI:10.1080/00397911.2010.538888.
[2] WANG Y. Potassium tert-Butoxide[J]. Synlett, 2011. DOI:10.1055/S-0031-1289873.
[3] B. S. BHAKUNI. Potassium tert‐Butoxide Mediated Synthesis of Phenanthridinone[J]. Organic Syntheses, 2014. DOI:10.1002/0471264229.OS090.16.
You may like
Related articles And Qustion
Lastest Price from Potassium tert-butoxide manufacturers

US $10.00/KG2025-04-21
- CAS:
- 865-47-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $6.00/kg2025-04-21
- CAS:
- 865-47-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month