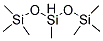Poly(methylhydrosiloxane): Overview and Conversion Method of its Waste to Useful Commodities
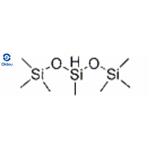
Poly(methylhydrosiloxane) (PMHS) is a polymer with the general structure [−CH3(H)Si−O−]. It is used in organic chemistry as a mild and stable reducing agent easily transferring hydrides to metal centers and a number of other reducible functional groups.

Preparation
Poly(methylhydrosiloxane) is prepared by the hydrolysis of monomethyldichlorosilane:
n MeSiHCl2 + n H2O → [MeSiHO]n + 2n HCl
The related polymer polydimethylsiloxane (PDMS) is made similarly, but lacking Si−H bonds, it exhibits no reducing properties. Dimethyldichlorosilane is then used instead of monomethyldichlorosilane.
Illustrative of its use, PMHS is used for in situ conversion of tributyltin oxide to tributyltin hydride:
2 MeSiH + (Bu3Sn)2O → Me2Si2O + 2 Bu3SnH
Uses
Poly(methylhydrosiloxane) (PMHS) is an easily han dled, inexpensive, non-toxic, and mild reducing agent. Although relatively inert towards organic functionality, PMHS can transfer its hydride to a variety of metalcatalysts (including Sn, Ti, Zn, Cu, and Pd) which can then participate in a wide range of reductions. Alternatively, when made hypercoordinate by the action of fluo ride or other nucleophiles, PMHS can act directly as a reducing agent. PMHS is attractive as a substitute for more expensive or hazardous silanes or siloxanes and as the stoichiometric reductant in catalytic organotin-mediated processes.1
References:
[1] Aldrichimica ACTA[J]. C&EN Global Enterprise, 2009, 87 48: 1-40. DOI:10.1021/cen-v087n048.p002.You may like
Related articles And Qustion
See also
Lastest Price from Poly(methylhydrosiloxane) manufacturers
US $0.00-0.00/KG2025-11-27
- CAS:
- 63148-57-2
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00/KG2025-06-12
- CAS:
- 63148-57-2
- Min. Order:
- 1KG
- Purity:
- 15-35cs
- Supply Ability:
- 200mt per month