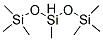Application of Poly(methylhydrosiloxane)
Description
Poly(methylhydrosiloxane) is a reducing agent. For example, it can be used for the reduction of esters to alcohols as well as the reduction of aldehydes and ketone. It can also be used for the reduction of phosphine oxides to phosphine. Recent study has also used it for conjugate reduction of α, β-Unsaturated Carbonyl and Carboxyl Compounds. Finally, it is also used in greener amine synthesis mediated by reductive amination which is an alternative to borohydrides.
Application
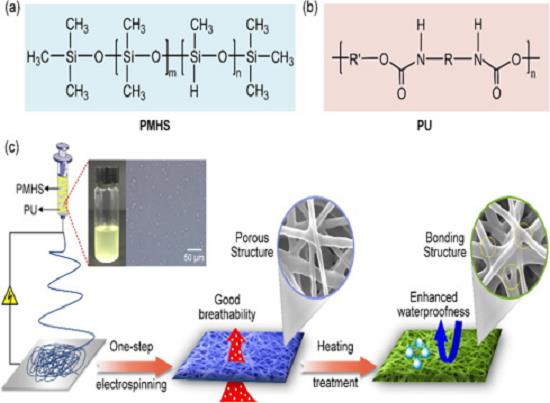
Polymethylhydrosiloxane(PMHS) is an easily handled,inexpensive,non-toxic,and mild reducing agent. PMHS is attractive as a substitute for more expensive or hazardous silanes or siloxanes and as the stoichiometric reductant in catalytic organotin-mediated processes. It can be cross linked by metal catalyst at low temperature to form waterproof membrane. It is used as waterproof agent of textile, glass, pottery, paper, leather, metal, cement and marble etc, especially in textile waterproof. It is used as insulator and cross linker of paper. Poly(methylhydrosiloxane) is used in the reduction of esters to alcohols, catalyzed by a combination of titanocene dichloride and either n-BuLi or EtMgBr. It is a safer alternative to triethoxysilane, B22063, for reduction of phosphine oxides to phosphines, catalyzed by Ti(O-i-Pr)4. It is also used in greener amine synthesis by reductive amination as an alternative to borohydrides.
Preparative Methods
hydrolysis of methyldichlorosilane followed by heating (60–150 ◦C) the resultant mixture of cyclicsilanes in the presence of hexamethyldisiloxane generates the linear polysiloxane.
Handling, Storage, and Precautions
stable to air and moisture; incompatible with strong acids, bases, or oxidants (forms hydrogen upon decomposition); generally considered non-toxic, however thorough toxicity studies have not been performed; skin/eye contact and inhalation should be avoided.
Conclusion
Polymethylhydrosiloxane (PMHS) is an easily handled, inexpensive, non-toxic, and mild reducing agent. Although relatively inert towards organic functionality, PMHS can transfer its hydride to a variety of metal catalysts (including Sn, Ti, Zn, Cu, and Pd) which can then participate in a wide range of reductions. Alternatively, when made hypercoordinate by the action of fluoride or other nucleophiles, PMHS can act directly as a reducing agent. PMHS is attractive as a substitute for more expensive or hazardous silanes or siloxanes and as the stoichiometric reductant in catalytic organotin-mediated processes.
You may like
Related articles And Qustion
Lastest Price from Poly(methylhydrosiloxane) manufacturers
US $0.00-0.00/KG2025-11-27
- CAS:
- 63148-57-2
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00/KG2025-06-12
- CAS:
- 63148-57-2
- Min. Order:
- 1KG
- Purity:
- 15-35cs
- Supply Ability:
- 200mt per month