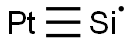Platinum silicide(PtSi):Crystal structure,Synthesis,Uses
Platinum silicide is an inorganic compound with the formula PtSi. It is a semiconductor that turns into a superconductor when cooled to 0.8 K.
Crystal structure
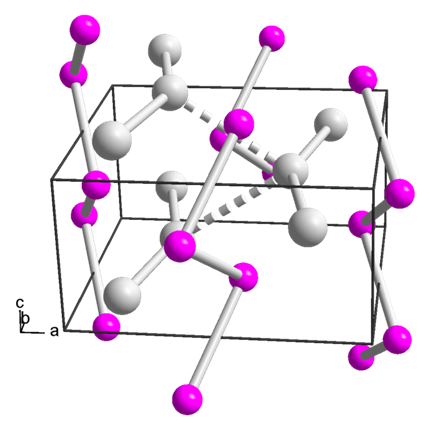
The crystal structure of Platinum silicide is orthorhombic, with each silicon atom having six neighboring platinum atoms.
One set is the three-center Pt–Si–Pt bond, and the other set is the two-center Pt–Si bond. Each silicon atom in the compound has one three-center bond and two-center bonds. The thinnest film of PtSi would consist of two alternating planes of atoms, a single sheet of orthorhombic structures.
Characteristic
Platinum silicide is capable of operating at 1-5 µm wavelength range. It has a good sensitivity (up to 0.05 °C) and high stability.
Synthesis
Platinum silicide(PtSi) can be synthesized in several ways. The standard method involves depositing a thin film of pure platinum onto silicon wafers and heating it in a conventional furnace at 450–600 ℃ for half an hour in inert ambients. The process cannot be carried out in an oxygenated environment, as this results in the formation of an oxide layer on the silicon, preventing PtSi from forming.
Uses
Platinum silicide is a semiconductor and a Schottky barrier with high stability and good sensitivity and can be used in infrared detection, thermal imaging, or ohmic and Schottky contacts.PtSi is now most commonly used in infrared detectors, due to the large size of wavelengths it can be used to detect.