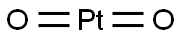Platinum Dioxide: Characteristics, Synthesis Method and Safety
General Description
Platinum dioxide, a rare compound, exhibits remarkable stability, catalytic activity, and electrical conductivity. Synthesized by heating platinum in an oxygen-rich environment, it demands careful handling to prevent reversion to platinum metal. Despite Platinum dioxide's utility, safety precautions are crucial due to its potential for fire, explosion, and irritation to eyes and skin. Proper storage, protective gear, and risk assessment are essential in occupational settings. Overall, while platinum dioxide's unique properties make it valuable for various applications, its safety considerations underscore the need for stringent protocols to ensure safe handling and usage.

Figure 1. Platinum Dioxide
Characteristics
Platinum dioxide, a rare and exceptional compound, boasts a distinctive array of properties that distinguish it within the realm of oxides. Primarily, its hallmark is its extraordinary stability, withstanding decomposition even under extreme conditions. This resilience stems from the robust bonds forged between platinum and oxygen atoms, rendering it an ideal candidate for deployment in high-temperature environments. Moreover, platinum dioxide showcases outstanding catalytic prowess, adept at fostering a diverse array of chemical reactions. Its catalytic efficacy is underpinned by its capacity to adsorb and activate reactant molecules, thereby augmenting the velocity and efficiency of chemical conversions. Additionally, platinum dioxide is renowned for its superior electrical conductivity, endowing it with considerable utility in electronic applications. Nonetheless, it is imperative to acknowledge that platinum dioxide remains relatively scarce and costly, constraining its widespread adoption. Nevertheless, its unparalleled attributes continue to captivate the interest of researchers and industrial stakeholders alike, as they endeavor to unlock its potential across a spectrum of applications. 1
Synthesis Method
The synthesis of Platinum Dioxide, commonly referred to as Platinum Oxide, primarily involves heating platinum metal in an oxygen-rich environment, typically at high temperatures. This method is the most prevalent and effective means of producing Platinum Dioxide. The chemical reaction involved can be represented as follows:2 Pt(s) + O2(g) → 2 PtO2(s). Where platinum (Pt) reacts with oxygen (O2) to form platinum dioxide (PtO2) as a solid product. The reaction occurs at elevated temperatures to facilitate the transformation of platinum metal into platinum dioxide. After the reaction is complete, the resulting platinum dioxide is allowed to cool before it is collected. It's essential to handle the product with care, as platinum dioxide is not stable at room temperature and tends to revert back to platinum metal over time in the absence of oxygen. Therefore, appropriate storage conditions, such as maintaining an oxygen-rich environment, may be necessary to preserve the integrity of the platinum dioxide. In summary, the synthesis of platinum dioxide involves heating platinum metal in the presence of oxygen to form platinum dioxide, which requires careful handling and storage to prevent its conversion back to platinum metal. 2
Safety
Platinum dioxide, while possessing certain industrial and scientific applications, demands careful handling and consideration due to its potential safety hazards. As indicated by its hazard labeling, platinum dioxide poses risks of fire, explosion, and severe irritation to the eyes and skin. Firstly, it serves as a strong oxidizer, implying that it can intensify fires and facilitate explosions. This characteristic demands strict adherence to proper storage and handling procedures to mitigate the risk of accidental ignition or combustion when in contact with combustible materials. Moreover, platinum dioxide is identified as a cause of serious eye irritation, indicating the necessity for appropriate eye protection measures when working with the compound. Additionally, it exhibits skin irritant properties and can potentially induce skin sensitization, warranting the use of protective clothing and gloves to prevent direct skin contact and allergic reactions. Furthermore, exposure to platinum dioxide may lead to associated occupational diseases, emphasizing the importance of implementing comprehensive safety protocols and conducting regular risk assessments in occupational settings where the compound is utilized. In summary, the safety of platinum dioxide hinges on meticulous adherence to handling guidelines, utilization of personal protective equipment, and continuous monitoring of occupational exposure levels to safeguard against potential hazards and associated health risks. 3
Reference
1. Platinum(IV) oxide. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 345198
2. Platinum Oxide. Material Poperties.
3. Platinum dioxide. European Chemicals Agency. EC/List no. 215-223-0.
Related articles And Qustion
Lastest Price from Platinum dioxide manufacturers

US $30.00-10.00/g2025-11-03
- CAS:
- 1314-15-4
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 300tons

US $1.00/KG2025-06-27
- CAS:
- 1314-15-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt