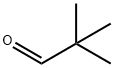
Pivaldehyde: applications and safety
General Description
Pivaldehyde exhibits unique applications in organic synthesis and pharmaceutical development. In multicomponent crotylation and acetal substitution reactions, pivaldehyde consistently yields the syn product, making it a key substrate for achieving specific diastereoselectivities. Computational studies reveal its distinct behavior can be attributed to the attack of O-methyl-substituted carboxenium ions by crotyl silane, indicating an S(N)1-type reaction mechanism via an ionic intermediate. Furthermore, pivaldehyde plays a crucial role in the synthesis of α-hydroxy-α-phenylamides, potent blockers of voltage-gated sodium channels, with promising potential in reducing prostate cancer. However, it is important to note that pivaldehyde is a highly flammable liquid and vapor, requiring careful handling and storage, as well as strict adherence to safety protocols to minimize health and environmental risks. Overall, pivaldehyde's significance in synthetic organic chemistry and pharmaceutical development is underscored by its diverse applications and unique reactivity.

Figure 1. Pivaldehyde
Applications
Crotylation
Pivaldehyde, a significant component in a study, demonstrates unique application in multicomponent crotylation (MCC) and acetal substitution (AS) reactions. Regardless of the substrate's double bond geometry, the crotylation of pivaldehyde consistently yields the syn product, distinguishing it from the behavior of acetaldehyde and propionaldehyde. This distinctive feature positions pivaldehyde as a key substrate for achieving specific diastereoselectivities in these reactions. Computational investigation utilizing the B3LYP/6-31+G(d) level of theory in dichloromethane solution reveals that the attack of O-methyl-substituted carboxenium ions by crotyl silane explains the observed selectivities, indicating an S(N)1-type reaction mechanism via an ionic intermediate. Moreover, the study rationalizes the observed selectivities by comparing relevant open transition-state structures, emphasizing the necessity of three transition-state conformations to determine selectivity. The research also delves into the activation energies for the stereogenic step, providing valuable insights into the mechanistic aspects of these reactions. Overall, the unique behavior of pivaldehyde in these reactions underscores its significance in predicting diastereoselectivity in general Lewis acid-mediated crotylations of aldehydes and ketones, thereby showcasing its pivotal role in synthetic organic chemistry. 1
Synthesis of α-hydroxy-α-phenylamides
Pivaldehyde, a key component in the synthesis of α-hydroxy-α-phenylamides, plays a crucial role in the development of small molecules that inhibit voltage-gated sodium channels. These channels, typically found in neurons and excitable cells, have also been identified in human prostate cancer cells. The α-hydroxy-α-phenylamides, characterized by a hydroxyamide motif which mimics a hydantoin ring, serve as potent blockers of these channels. In order to optimize the efficacy of these sodium channel blockers, it is essential to consider the chiral preferences of the synthesized compounds. By utilizing Seebach and Frater's chiral template, researchers were able to perform cyclocondensation of (R)-3-chloromandelic acid with pivaldehyde to produce both cis- and trans-2,5-disubsituted dioxolanones. Subsequently, this chiral template facilitated the synthesis of both enantiomers of 2-(3-chlorophenyl)-2-hydroxynonanamide, which were then evaluated for their ability to functionally inhibit hNa(v) isoforms, human prostate cancer cells, and xenograft. The results showed that the enantiomers derived from pivaldehyde demonstrated significant potential in reducing prostate cancer in vivo, highlighting the promising application of pivaldehyde in the development of novel treatments for prostate cancer targeting voltage-gated sodium channels. 2
Safety
Pivaldehyde is a chemical compound with important industrial applications. It is crucial to understand the safety considerations associated with pivaldehyde due to its hazardous nature. Pivaldehyde is classified as a highly flammable liquid and vapor, posing a significant fire and explosion risk. As such, it should be stored and handled with extreme caution in well-ventilated areas, away from any potential sources of ignition. In addition to its flammability, pivaldehyde can cause skin and respiratory irritation upon contact or inhalation. Therefore, appropriate personal protective equipment such as gloves and respiratory protection should be worn when working with this compound. Furthermore, exposure to pivaldehyde should be minimized, and strict adherence to safety protocols and guidelines is essential to prevent accidents and health risks. Proper training, risk assessment, and emergency response procedures should be in place to ensure the safe handling and use of pivaldehyde in industrial settings. 3
Reference
1. Tietze LF, Kinzel T, Schmatz S. Origin of syn/anti diastereoselectivity in aldehyde and ketone crotylation reactions: a combined theoretical and experimental study. J Am Chem Soc. 2006 Sep 6;128(35):11483-11495.
2. Davis GC, Kong Y, Paige M, Li Z, Merrick EC, Hansen T, Suy S, Wang K, Dakshanamurthy S, Cordova A, McManus OB, Williams BS, Chruszcz M, Minor W, Patel MK, Brown ML. Asymmetric synthesis and evaluation of a hydroxyphenylamide voltage-gated sodium channel blocker in human prostate cancer xenografts. Bioorg Med Chem. 2012 Mar 15;20(6):2180218-8.
3. Pivalaldehyde. European Chemicals Agency, EC / List no. 211-134-6.
Related articles And Qustion
Lastest Price from Pivaldehyde manufacturers
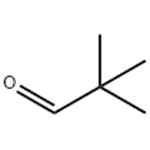
US $0.00/kg2025-10-28
- CAS:
- 630-19-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00/kg2025-09-26
- CAS:
- 630-19-3
- Min. Order:
- 1kg
- Purity:
- 96%
- Supply Ability:
- 1