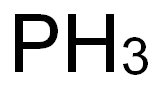Phosphorus: Production, application, toxicity and biological role
General description
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Earth. It has a concentration in the Earth's crust of about one gram per kilogram (compare copper at about 0.06 grams). In minerals, phosphorus generally occurs as phosphate. Phosphorus is essential for life. Phosphates (compounds containing the phosphate ion, PO43−) are a component of DNA, RNA, ATP, and phospholipids. Elemental phosphorus was first isolated from human urine, and bone ash was an important early phosphate source. Its appearance is as follows:

Figure 1 Appearance of Phosphorus.
Production
Most production of phosphorus-bearing material is for agriculture fertilisers. For this purpose, phosphate minerals are converted to phosphoric acid. It follows two distinct chemical routes, the main one being treatment of phosphate minerals with sulfuric acid. The other process utilises white phosphorus, which may be produced by reaction and distillation from very low grade phosphate sources. The white phosphorus is then oxidised to phosphoric acid and subsequently neutralised with base to give phosphate salts. Phosphoric acid produced from white phosphorus is relatively pure and is the main route for the production of phosphates for all purposes, including detergent production. In the early 1990s, Albright and Wilson's purified wet phosphoric acid business was being adversely affected by phosphate rock sales by China and the entry of their long-standing Moroccan phosphate suppliers into the purified wet phosphoric acid business.
Application
Phosphorus is an essential plant nutrient (the most often limiting nutrient, after nitrogen),[1] and the bulk of all phosphorus production is in concentrated phosphoric acids for agriculture fertilisers, containing as much as 70% to 75% P2O5. That led to large increase in phosphate (PO43−) production in the second half of the 20th century. Artificial phosphate fertilisation is necessary because phosphorus is essential to all living organisms; it is involved in energy transfers, strength of root and stems, photosynthesis, the expansion of plant roots, formation of seeds and flowers, and other important factors effecting overall plant health and genetics.[1]
White phosphorus is widely used to make organophosphorus compounds through intermediate phosphorus chlorides and two phosphorus sulfides, phosphorus pentasulfide and phosphorus sesquisulfide. Organophosphorus compounds have many applications, including in plasticisers, flame retardants, pesticides, extraction agents, nerve agents and water treatment.[2] Phosphorus is also an important component in steel production, in the making of phosphor bronze, and in many other related products. Phosphorus is added to metallic copper during its smelting process to react with oxygen present as an impurity in copper and to produce phosphorus-containing copper (CuOFP) alloys with a higher hydrogen embrittlement resistance than normal copper.[3]
Toxicity
Organic compounds of phosphorus form a wide class of materials; many are required for life, but some are extremely toxic. Fluorophosphate esters are among the most potent neurotoxins known. A wide range of organophosphorus compounds are used for their toxicity as pesticides (herbicides, insecticides, fungicides, etc.) and weaponised as nerve agents against enemy humans. Most inorganic phosphates are relatively nontoxic and essential nutrients.[2] The white phosphorus allotrope presents a significant hazard because it ignites in air and produces phosphoric acid residue. Chronic white phosphorus poisoning leads to necrosis of the jaw called "phossy jaw". White phosphorus is toxic, causing severe liver damage on ingestion and may cause a condition known as "Smoking Stool Syndrome".
Biological role
Inorganic phosphorus in the form of the phosphate PO3−4 is required for all known forms of life. Phosphorus plays a major role in the structural framework of DNA and RNA. Living cells use phosphate to transport cellular energy with adenosine triphosphate (ATP), necessary for every cellular process that uses energy. ATP is also important for phosphorylation, a key regulatory event in cells. Phospholipids are the main structural components of all cellular membranes. Calcium phosphate salts assist in stiffening bones.[2] Biochemists commonly use the abbreviation "Pi" to refer to inorganic phosphate.[4]
References
[1]Etesami, H. (2019). Nutrient Dynamics for Sustainable Crop Production. p. 217.
[2]Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
[3]Joseph R. Davisz, ed. (January 2001). Copper and Copper Alloys. ASM International. p. 181. ISBN 0871707268.
[4]Lipmann, D (1944). Enzymatic Synthesis of Acetyl Phosphate. J Biol Chem. 155: 55–70.You may like
Related articles And Qustion
Lastest Price from Phosphorus manufacturers

US $0.00/kg2024-12-17
- CAS:
- 7723-14-0
- Min. Order:
- 10kg
- Purity:
- 99% purity
- Supply Ability:
- 1000 kg

US $9.00/KG2024-05-29
- CAS:
- 7723-14-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000tons