Pharmacological activities of Schizandrol A
Introduction
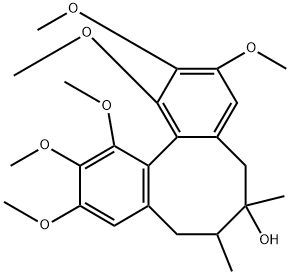
Schisandra lignans are the main active ingredients in schisandra, and schisandrol A (Figure 1) is the representative ingredient, the standard reference used for the identification and quality evaluation of schisandra, with some pharmacological activities such as antitumor and prevention of Alzheimer's Disease. This report summarized the pharmacological action of schizandrol A.

Chemistry
Schizandrol A or Schisandrin (systematic name: 1,2,3,10,11,12-hexamethoxy-6,7-dimethyl-5,6,7,8-tetrahydrodibenzo[a,c]cycloocten-6-ol), C24H32O7, has a dibenzocyclooctadiene skeleton.There are three molecules in the asymmetric unit, which are related by a pseudo-translation in the direction of the c axis.Nevertheless, the three molecules differ in the torsion angle of one of the methoxy groups. The dihedral angles between the two aromatic rings are 62.39 (10), 62.65 (10) and 61.84 (10) for the three molecules. The crystal packing is stabilized by a series of O-H···O and C-H···O hydrogen bonds, as well as C-H···π interactions.[1]
Pharmacological activities
Pharmacokinetics and distribution of schisandrol A and its majormetabolites in rats
A rapid resolution liquid chromatography coupled with quadruple-time-of-flight mass spectrometry (RRLC-QTOF/MS) was developed to investigate the pharmacokinetics of schizandrol A after its intragastric administration (50 mg/kg) in rats. Schizandrol A was rapidly absorbed (Tmax = 2.07 h), with a longer duration (t1/2= 9.48 h) and larger apparent volume of distribution (Vz/F = 111.81 l/kg) in rats. Schizandrol A can be detected in main organs and the order of its distribution was in the liver > kidney > heart > spleen > brain, particularly higher in the liver. Five schizandrol A metabolites were identified, including 2-demethyl-8(R)-hydroxyl-schizandrin, 3-demethyl-8(R)-hydroxyl-schizandrin, hydroxyl-schizandrin, demethoxy-schizandrin, 2, 3-demethyl-8(R)-hydroxyl-schizandrin, indicating that the hydroxylation and demethylation may be the major metabolic way of schizandrol A. This study defined the pharmacokinetic characteristics of schizandrol A in vivo, and the RRLC-QTOF/MS is more sensitive and less limited by conditions, and needs less samples, which may be a useful resource for the further research and development of schisandrol A.[2]
Schizandrol A reverses multidrug resistance in resistant chronic myeloid leukemia cells K562/A02
Overexpression of P-gp is the main cause of multidrug resistance (MDR) and chemotherapeutic failure in leukemia. In this study, the multidrug resistance reverse effect of Schizandrol A (SchA) was demonstrated with P-gp overexpressed drug-resistant K562/A02 cells. Schizandrol A had almost no cytotoxic activity, the EC50 value reversed to DOX was in the nanomole range of (707±29nM) and had a high selectivity index (>113) to normal cells. DOX accumulation and Rh123 efflux tests demonstrated that the MDR reversal activity of Schizandrol A was induced by inhibiting P-gp function. Western blotting assay showed that Schizandrol A down-regulated the expression of P-gp by inhibiting the PI3K/Akt signaling pathway, which was also a key factor in reversal activity. In conclusion, Schizandrol A not only improved the drug resistance of K562/A02 to chemotherapeutic drug DOX by inhibiting P-gp function, but also achieved reverseactivity by down-regulating P-gp expression through PI3K/Akt signaling pathway. Schizandrol A can be used as a potential drug candidate for natural P-gp mediated MDR reversal agents.[3]
Schizandrin A can inhibit non-small cell lung cancer cell proliferation by inducing cell cycle arrest, apoptosis and autophagy
Schizandrin A (SchA) can be extracted from the vine plant Schisandra chinensis and has been reported to confer various biologically active properties. However, its potential biological effects on non small cell lung cancer (NSCLC) remain unknown. Therefore, the present study aims to address this issue. NSCLC and normal lung epithelial cell lines were first treated with Schizandrol A. Cell viability and proliferation were measured using CellTiter Glo Assay and colony formation assays, respectively. PI staining was used to measure cell cycle distribution. Cell cycle related proteins p53, p21, cyclin D1, CDK4, CDK6, cyclin E1, cyclin E2, CDK2 and DNA damage related protein SOX4 were detected by western blot analysis. Annexin VFITC/PI staining, DNA electrophoresis and Hoechst 33342/PI dual staining were used to detect apoptosis. JC1 and DCFHDA fluorescent dyes were used to measure the mitochondrial membrane potential and reactive oxygen species concentrations, respectively. Apoptosis related proteins caspase3, cleaved caspase3, poly(ADP ribose) polymerase (PARP), cleaved PARP, BimEL, BimL, BimS, Bcl2, Bax, caspase9 and cleaved caspas9 were measured by western blot analysis. Dansylcadaverine was used to detect the presence of the acidic lysosomal vesicles. The expression levels of the autophagy related proteins LC3I/II, p62/SQSTM and AMPKα activation were measured using western blot analysis. In addition, the autophagy inhibitor 3-methyladenine was used to inhibit autophagy. Schizandrol A treatment was found to reduce NSCLC cell viability whilst inhibiting cell proliferation. Low concentrations of Schizandrol A (10-20 µM) mainly induced G 1/S phase cell cycle arrest. By contrast, as the concentration of SchA used increases (20-50 µM), cells underwent apoptosis and G 2/M phase cell cycle a13rrest. As the treatment concentration of Schizandrol A increased from 0 to 50 µM, the expression of p53 and SOX4 protein also concomitantly increased, but the expression of p21 protein was increased by 10 µM SchA and decreased by higher concentrations (20-50 µM). In addition, the mRNA and protein expression levels of Bcl-like 11 (Bim)EL, BimL and BimS increased following SchA application. SchA induced the accumulation of acidic vesicles and induced a marked increase in the expression of LC3-II protein, suggsting that Schizandrol A activated the autophagy pathway. However, the expression of the p62 protein was found to be increased by Schizandrol A, suggesting that p62 was not degraded during the autophagic flux. The 3-methyladenine exerted no notable effects on SchA induced apoptosis. Taken together, results from the present study suggest that Schizandrol A exerted inhibitory effects on NSCLC physiology by inducing cell cycle arrest and apoptosis. In addition, Schizandrol A partially induced autophagy, which did not result in any cytoprotective effects.[4]
Exploring the protective effects of schizandrol A in acute myocardial ischemia mice Schizandrol A has been used as a remedy to prevent oxidative injury. However, whether the cardioprotective effect of schizandrol A is associated with regulating endogenous metabolites remains unclear, thus the researchers performed comprehensive metabolomics profiling in acute myocardial ischemia (AMI) mice following schizandrol A treatment. AMI was induced in ICR mice by coronary artery ligation, then schizandrol A (6 mg·kg-1/d, ip) was administered. Schizandrol A treatment significantly decreased the infarct size, preserved the cardiac function, and improved the biochemical indicators and cardiac pathological alterations. Moreover, schizandrol A (10,100 M) significantly decreased the apoptotic index in OGD-treated H8c2 cardiomycytes in vitro. By using HPLC-Q-TOF/MS, we conducted metabonomics analysis to screen the significantly changed endogenous metabolites and construct the network in both serum and urine. The results revealed that schizandrol A regulated the pathways of glycine, serine and threonine metabolism, lysine biosynthesis, pyrimidine metabolism, arginine and proline metabolism, cysteine and methionine metabolism, valine, leucine and isoleucine biosynthesis under the pathological conditions of AMI. Furthermore, the researchers selected the regulatory enzymes related to heart disease, including ecto-5'-nucleotidase (NT5E), guanidinoacetate N-methyltransferase (GAMT), platelet-derived endothelial cell growth factor (PD-ECGF) and methionine synthase (MTR), for validation.In addition, schizandrol A was found to facilitate PI3K/Akt activation and inhibit the expression of NOX2 in AMI mice and OGD-treated H9c2 cells. In conclusion, the researchers have elucidated SA-regulated endogenous metabolic pathways and constructed a regulatory metabolic network map. Furthermore, the researchers have validated the new potential therapeutic targets and underlying molecular mechanisms of schizandrol A against AMI, which might provide a reference for its future application in cardiovascular diseases.[5]
References
[1]Zhao L, Yu X, Chen C. Schizandrol A: a lignan from Schisandra chinensis. Acta Crystallogr Sect E Struct Rep Online. 2008;64(Pt 8):o1514. Published 2008 Jul 16. doi:10.1107/S1600536808021545
[2]Liu X, Cong L, Wang C, et al. Pharmacokinetics and distribution of schisandrol A and its major metabolites in rats. Xenobiotica. 2019;49(3):322-331. doi:10.1080/00498254.2017.1418543
[3]Arken N. Schizandrol A reverses multidrug resistance in resistant chronic myeloid leukemia cells K562/A02. Cell Mol Biol (Noisy-le-grand). 2019;65(1):78-83. Published 2019 Jan 31.
[4] Zhu L, Wang Y, Lv W, et al. Schizandrin A can inhibit non-small cell lung cancer cell proliferation by inducing cell cycle arrest, apoptosis and autophagy. Int J Mol Med. 2021;48(6):214. doi:10.3892/ijmm.2021.5047
[5]Lai Q, Yuan GY, Wang H, et al. Exploring the protective effects of schizandrol A in acute myocardial ischemia mice by comprehensive metabolomics profiling integrated with molecular mechanism studies. Acta Pharmacol Sin. 2020;41(8):1058-1072. doi:10.1038/s41401-020-0377-7
You may like
See also
Lastest Price from Schisandrin manufacturers

US $1200.00-1100.00/ton2025-10-21
- CAS:
- 7432-28-2
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $5.00-0.50/KG2025-05-08
- CAS:
- 7432-28-2
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS