Pharmacological action and Preparation of Arbutin
General description
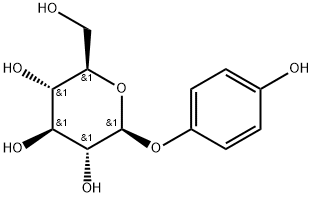
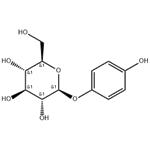
Arbutin is a beta-D-glucopyranoside extracted from the dried leaves of bearberry plant in the genus Arctostaphylos and other plants commonly in the Ericaceae family. CAS number is 497-76-7. It is found in foods, over-the-counter drugs, and herbal dietary supplements and its chemical name is p-hydroxybenzene-D-glucopyranoside (Figure 1), which is a white needle-like crystal, easily soluble in hot water, methanol, ethanol and aqueous solutions of propylene glycol and glycerol, but insoluble in solvents such as ether, chloroform, petroleum ether, etc [1]. Its chemical’s molecular formula is C12H16O7 and molecular weight is 272.251. Arbutin is widely used in the cosmetics and pharmaceutical industry due to its whitening and pigment removing properties, and pharmacological effects such as antioxidant, anti-microbial and anti-inflammatory activities. In recent years, it has also been used in improving wine aroma and other food. As people′s emphasis on diet and health care, many drugs also develop usage in food[2].
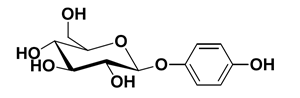
Figure 1 The molecular formula of Arbutin
Uses
Indicated for over-the-counter use for epidermal hyperpigmentation in various skin conditions, such as melasma, freckles, and senile lentigines[3].
Arbutin was originally a natural active substance extracted from the bearberry tree, which can remove freckles, butterfly spots and seborrheic acne on the skin. In recent years, many countries have used arbutin as a whitening agent in cosmetics to inhibit the production of melanin in skin cells, so as to achieve the effect of whitening the skin, and the effect is outstanding.
Compared with other traditional whitening agents (such as mercury salt, azelaic acid, etc.), arbutin has the advantages of safety, non toxicity, good freckle removing effect, convenient use, etc. it is one of the skin whitening agents with high decolorization efficiency, safety, reliability and good compatibility
As an ideal skin-whitening and freckle-removing active agent in the 21st century, arbutin is a highly effective depigmentation component that integrates "green plants, safety and reliability". Arbutin has good hydrophilicity while protecting the skin from free radicals, so it is often sold in the market as a whitening agent and is very popular in Asian countries. With the deepening of research and the further expansion of research scope in recent years, more effects of arbutin have been highlighted, such as antioxidant, anti-inflammatory, antibacterial, antitussive, expectorant, antiasthmatic, anticancer, antitumor, therapeutic acute lung injury, etc[4]. Therefore, the demand for arbutin in the cosmetic market and the pharmaceutical market is increasing year by year.
Pharmacological action
Inhibit melanin.
As a tyrosinase activity competition inhibitor, arbutin is reversible. It can quickly penetrate into the skin, effectively inhibit tyrosinase activity within the concentration range of non-toxic melanocytes and without affecting the concentration of cell proliferation. Through its direct binding with tyrosinase, arbutin competes for the binding site of dopa, blocks the synthesis of dopa and DOPA quinone, and then interferes with melanocytes and inhibits the production of melanin. At the same time, it also has the function of desalinating the formed melanin, accelerating the decomposition and excretion of melanin, reducing skin pigment deposition, and removing spots and freckles.
Analgesia, anti inflammation and bacteriostasis.
Arbutin can be used for anti-inflammatory and anti ulcer. Pharmacologically, it can inhibit the degradation of insulin. After oral administration, it hydrolyzes in vivo to produce hydroquinone and has bactericidal effect. In traditional medical treatment, arbutin, as the main pharmacodynamic component of arbutin leaves, is mainly used to treat urinary tract infection and intestinal inflammation with its strong bactericidal and anti-inflammatory ability. Studies have shown that arbutin can significantly reduce pro-inflammatory cytokines and other inflammation related genes. It can be used to treat the inflammation and harmful effects of BV2 microglia activation stimulated by lipopolysaccharide (LPS). It can quickly relieve pain, quickly eliminate swelling, heal quickly, leave no scars, and have no side effects.
Antitussive, expectorant and asthma.
Cough, phlegm and asthma are the three common symptoms of respiratory diseases. Chronic recurrent cough, expectoration or wheezing are the main clinical manifestations of chronic nonspecific inflammation of tracheal and bronchial mucosa and its surrounding tissues. Studies have shown that arbutin can increase tracheal secretion, prolong the incubation period of cough induced by ammonia, reduce the number of cough, and significantly increase the amount of phenol red excretion in the trachea[5,6].
Anticancer.
The treatment of tumor with traditional Chinese medicine extract is a hot spot of current research. It has been clinically confirmed that arbutin has no obvious toxic and side effects in the human body, and some studies have shown the effect of arbutin as an antioxidant on prostate cancer cells. It has been found that arbutin can significantly induce tumor cell apoptosis, opening up a new prospect for the adjuvant treatment of prostate cancer[7]. At the same time, it also shows that arbutin has potential anti-tumor effect and can effectively inhibit the metastasis behavior of some cancer cells.
Antioxidant.
As an antioxidant, arbutin can reduce oxidation level parameters, including lipid peroxidation markers, nitrite, protein carbonyl and iron reduction antioxidant capacity, improve SOD and glutathione peroxidase activity, reduce oxidative stress and nitrous stress, reduce MDA content, reduce ROS level and enhance antioxidant status. The antioxidant effect of arbutin can be applied to the nervous system, cardiovascular system and immune system to maintain the overall health of the body.
Mechanism of action[8]
Arbutin is a hydroquinone glycoside, however the hydroquinone moiety is not solely responsible for the de-pigmentating actions of arbutin. It acts as a competitive inhibitor of tyrosinase enzyme by acting on the L-tyrosine binding site to suppress melanogenesis and mediate its de-pigmenting actions on human skin 1. Tyrosinase is an enzyme involved in the regulation of rate-limiting steps during the synthesis of melanin; it regulates the conversion of L-tyrosine into L-dopa, and subsequent conversion of L-dopa to L-dopaquinone 1. Via inhibition of tyrosinase activity in a concentration-dependent manner, arbutin attenuates the production of melanin in melanocytes. Arbutin may also downregulate the expression of tyrosinase in addition to its inhibitory action on the enzyme.
Toxicity[3]
In an acute oral toxicity study, the LD50-value for β-arbutin is 9804 mg/kg bw for the mouse and 8715 mg/kg bw for the rat. Dermal LD50 value in rat and mouse was reported to be greater than 928 mg/kg bw, according to an acute dermal toxicity study. Extremely high doses may cause ringing in the ears, shortness of breath, convulsions, collapse, vomiting and delirium. Nausea and vomiting were seen individuals with sensitive stomachs following oral ingestion of 15 g of dried uva ursi leaves that contain arbutin.
Preparation
Natural plant extraction.
As a naturally occurring active substance, arbutin was first extracted from the leaves of arbutin. However, due to the low content of arbutin in plants, the extraction process is particularly complex, and the purity of the extracted product is low, so the extraction cost is relatively high. With the development of other preparation methods, the plant extraction method has gradually lost its competitive advantage.
Plant tissue culture.
Plant tissue culture method uses the glycosylation ability of plant cells to convert hydroquinone into arbutin. Compared with plant extraction, arbutin obtained by plant tissue culture is much more efficient, but it also has shortcomings such as long production cycle, low content, and difficulty in subsequent separation.
Enzymatic conversion method.
The enzymatic conversion method mainly uses glycosyltransferase or glycosidase as catalyst to catalyze glycosyl transfer and reverse hydrolysis to synthesize glycosides. Therefore, arbutin can also be obtained from hydroquinone and glucose under the catalysis of glycosidase.
Organic synthesis.
Glycosidation reaction is the key to the synthesis of arbutin. According to the different glycosyl donors in the reaction, it can be divided into two kinds of synthesis methods. Scheme 1. A synthetic method using glycosyl bromoglycosides as donors (Koenigs Knorr method). Scheme 2. Synthesis method with acyl group as donor (Helferich method).
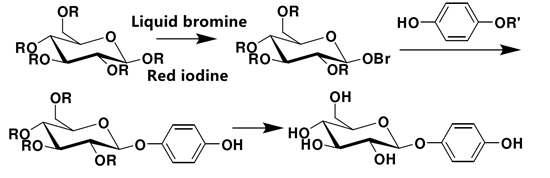
Scheme 1. Synthesis of arbutin by Koenigs Knorr method
Koenigs Knorr method requires expensive silver salt with high manufacturing cost. It is generally used for laboratory synthesis and not for industrial production.
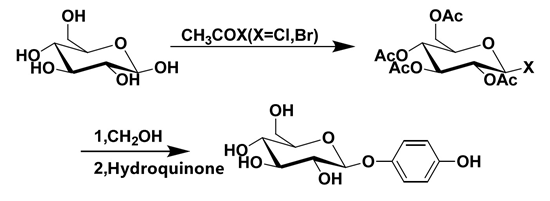
Scheme 2. Synthesis of arbutin by Helferich method
At present, the industrial synthesis of arbutin is mostly based on Helferich method. Helferich method initially used pentaacetyl glucose and phenol as raw materials to carry out glycosidation reaction under the action of zinc chloride or p-toluene sulfonic acid[10]. After continuous improvement, it has become one of the better methods to synthesize glycosides.
References
1. LIU Xiaoting, WANG Xinxuan.Research progress on the pharmacological action and mechanism of arbutin[J]. Food and Fermentation Industries,2022,48(02):309-316.
2. Arbutin suppresses osteosarcoma progression via miR-338-3p/MTH-FD1L and inactivation of the AKT/mTOR pathway[J].FEBS Open Bio,2021,11(1):289-299.
3. National Center for Biotechnology Information. "PubChem Compound Summary for for CID 440936, Arbutin" PubChem.
4. PARK J J,HWANG S J,KANG Y S,et al.Synthesis of Arbutingold nanoparticle complexes and their enhanced performance for whitening[J].Archives of Pharmacal Research,2019,42 (11):977-989.
5. TANG J Y,PENG F.Advances in pharmacological effects of arbutin and access to resources[J].Pharmacy Today,2015,2 (9):673-677.
6. ZHENG X K,BI Y F,FENG W S,et al.Study on the chemical constituents ofCyprinus scrolls[J].Acta Pharmaceutica Sinica,2004,39(4):266-268.
7. HAJAR S,EBRAHIM Z,MAHDI P,et al.Decrease of intracellular ROS by arbutin is associated with apoptosis induction and downregulation of IL-1βand TNF-αin LNCaP;prostate cancer[J].Journal of Food Biochemistry,2020,44(9):e13360.
8. Inoue Y, Hasegawa S, Yamada T, Date Y, Mizutani H, Nakata S, Matsunaga K, Akamatsu H: Analysis of the effects of hydroquinone and arbutin on the differentiation of melanocytes. Biol Pharm Bull. 2013;36(11):1722-30.
9. Liu Feng, Jiang Tao, Ren Sumei. Development in synthesis of arbutin[J]. China Surfactant Detergent & Cosmetics, 2004(04):242-244.
10. Huang S L, Zhu Y L, Pan Y-J, et al. Synthesis of arbutin by two-step reaction from glucose[J].J .Zhejiang Univ., 2004, 5(12):1509.
You may like
Related articles And Qustion
See also
Lastest Price from Arbutin manufacturers

US $0.00-0.00/kg2025-12-05
- CAS:
- 497-76-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg
US $0.00-0.00/kg2025-08-21
- CAS:
- 497-76-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1