Pharmacokinetics of amoxicillin trihydrate
Introduction
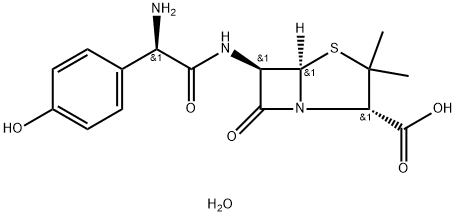
Amoxicillin trihydrate is a white to off-white free flowing crystalline powder. Amoxicillin is an antibiotic often used for the treatment of a number of bacterial infections. Amoxicillin trihydrate is a hydrate that is the trihydrate form of amoxicillin; a semisynthetic antibiotic, used either alone or in combination with potassium clavulanate (under the trade name Augmentin) for treatment of a variety of bacterial infections. It has a role as an antibacterial drug and an antimicrobial agent.
Properties
Amoxicillin trihydrate is used to treat many different types of infections caused by bacteria, such as ear infections, bladder infections, pneumonia, gonorrhea, and E. coli or salmonella infection. Amoxicillin trihydrate is also sometimes used together with another antibiotic called clarithromycin (Biaxin). Amoxicillin trihydrate is a white or almost white, crystalline powder, slightly soluble in water as shown in Table 1, very slightly soluble in alcohol, practically insoluble in fatty oils. It dissolves in dilute acids and dilute solutions of alkali hydroxide. Amoxicillin trihydrate does not actually kill bacteria, but instead it prevents them from multiplying, thus, making it easier for the immune system to wipe out the infection. It does this by stopping the new bacteria from forming their cell walls, which are necessary to avoid the contents of the bacterial cell from spewing out into its surroundings.
Crystalline amoxicillin trihydrate powder
The invention relates to crystalline amoxicillin trihydrate powder having a bulk density higher than 0.45 g/ml[4]. The invention also relates to a process for preparing crystalline amoxicillin trihydrate powder, said process comprising: crystallizing amoxicillin trihydrate from a solution containing dissolved amoxicillin; separating the crystals from said solution; and drying the separated crystals, resulting in crystalline powder having a bulk density higher than 0.45 g/ml.

Picture 1 amoxicillin trihydrate powder
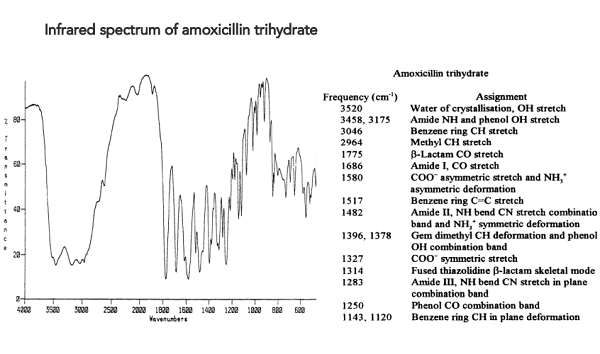
The stability of amoxicillin trihydrate
The effect of various environmental factors on the stability of aqueous solutions of amoxicillin-clavulanic acid combination in a veterinary water-soluble powder product was investigated. In the swine industry, the combination is administered via the drinking water, where both substances are quickly decomposed depending on several environmental factors. The degradation rate of the substances was determined in solutions of different water hardness levels (German hardness of 2, 6 and 10) and pH values (3.0, 7.0 and 10.0), and in troughs made of different materials (metal or plastic). Increasing the water hardness decreased the stability of both substances, amoxicillin being more stable at each hardness value than clavulanate. Amoxicillin trihydrate proved to be most stable at an acidic pH, while increasing the pH decreased its stability (P < 0.05).
Pharmacokinetics of amoxicillin trihydrate
The pharmacokinetics of amoxicillin were studied in five Desert sheep and five Nubian goats after intravenous (i.v.) or intramuscular (i.m.) administration of a single dose of 10 mg/kg body weight[2]. Following i.v. injection, the plasma concentration-versus-time data were best described by a two-compartment open model. The kinetic variables were similar in both species except for the volume of the central compartment (Vc), which was larger in sheep (p<0.05). Following i.m. injection, except for the longer half-life time of absorption in goats (p<0.05), there were no significant differences in other pharmacokinetic parameters between sheep and goats. The route of amoxicillin administration had no significant effect on the terminal elimination half-life in either species. The bioavailability of the drug (F) after i.m. administration was high (>0.90) in both species. These results indicate that the pharmacokinetics of amoxicillin did not differ between sheep and goats; furthermore, because of the high availability and short half-life of absorption, the i.m. route gives similar results to the i.v. route. Therefore, identical intramuscular and intravenous dose regimens should be applicable to both species.
A process for the preparation of amoxicillin trihydrate
The invention relates to an in-situ process for the preparation of amoxicillin trihydrate from sugarcane juice without isolation of the intermediates produced during the reaction sequence viz penicillin G and 6-aminopenicillanic acid[3]. The invention provides an in-situ cost effective and environment friendly process in which no separate synthesis of the intermediates, no purification cost and no crystallization cost for the intermediates is required.
Reference
1 Jerzsele á, Nagy G. The stability of amoxicillin trihydrate and potassium clavulanate combination in aqueous solutions[J]. Acta Veterinaria Hungarica, 2009, 57(4): 485-493.
2 Elsheikh H A, Taha A A, Khalafalla A E, et al. Pharmacokinetics of amoxicillin trihydrate in Desert sheep and Nubian goats[J]. Veterinary Research Communications, 1999, 23(8): 507-514.)
3 WO2014128538A1 A PROCESS FOR THE PREPARATION OF AMOXICILLIN TRIHYDRATE)
4 US2006166958A1 Crystalline amoxicillin trihydrate powder)
Related articles And Qustion
See also
Lastest Price from Amoxicillin trihydrate manufacturers
US $0.00-0.00/KG2025-11-25
- CAS:
- 61336-70-7
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS
US $0.10/KG2025-09-11
- CAS:
- 61336-70-7
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 1000 tons