Perfluoro(2-methyl-3-pentanone):environmental, implications and application research
Introduction
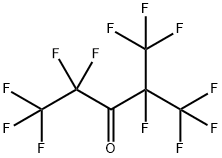
Perfluoro(2-methyl-3-pentanone) (PFMP; Figure 1) is a fire protection fluid, marketed as Novec 1230 by 3M. It is a replacement for chlorofluorocarbons (CFCs) and Halons, which deplete stratospheric ozone. The atmospheric lifetime of Perfluoro(2-methyl-3-pentanone) from previous work seems to be determined by photolysis and is approximately 1 week. Perfluoro(2-methyl-3-pentanone) does not contribute to stratospheric ozone depletion and has a negligible global warming potential. The major photolysis products are CF3C(O)F and COF2. The atmospheric fate of CF3C(O)F is hydrolysis to yield TFA. It is possible that chemistry in remote environments following the photolysis of PFMP would give small yields of PFPrA or isoperfluorobutanoic acid (i-PFBA) via reactions of the corresponding perfluoroacyl radicals with HO2 radicals.In addition to photolysis, hydration or abiotic hydrolysis maybe a significant sink of perfluoro(2-methyl-3-pentanone) in the environment. In the hydration reaction, perfluoro(2-methyl-3-pentanone) would react either reversibly or irreversibly with water to form a geminal diol which would shut down the photolysis pathway. Ketones are typically unreactive toward hydrolysis because the leaving group after nucleophilic attack is an aliphatic carbanion. Carbanions are highly basic and are not good leaving groups. However, hydrolysis of perfluoro(2-methyl-3-pentanone) gives a perfluorinated carbanion which, because the fluorine atoms stabilize the departing carbanion through hyperconjugation, is a much better leaving group.[1]

Environmental implications
Jackson and his coworkers have investigated the potential for photolysis, hydrolysis, and hydration to contribute to the environmental fate of perfluoro(2-methyl-3-pentanone). As discussed in the previous section, hydration is not a significant fate for perfluoro(2-methyl-3-pentanone). While the rate constant for hydrolysis is much greater than that for photolysis, the levels of liquid water in the atmosphere are usually very low. A typical cloud only contains approximately 3*10-7 cm3 liquid water per mL of total volume and we conclude that even at night, the amount of PFPrA and HFC-227ea produced would not be significant and that photolysis dominates hydrolysis as the atmospheric fate of perfluoro(2-methyl-3-pentanone). Cahill and Mackay came to the same conclusion in their modeling study. Interestingly, Cahill and Mackay predicted a hydrolysis rate constant at pH 5.6 of 2.2 s-1 which is approximately 104 times larger than the hydrolysis rate constant they measured. Hence, these work suggests hydrolysis is an even less important fate for perfluoro(2-methyl-3-pentanone) compared to photolysis than the ratio Cahill and Mackay predicted; the ratio of rates of photolysis to hydrolysis is approximately 980,000,000:1. For full details on this calculation, please see the SI. Photolysis will always dominate over hydrolysis. It is clear the very low fraction of liquid water in the atmosphere contributes heavily to this ratio and more than offsets the higher hydrolysis rate constant. This ratio is so great that even at night time during a heavy rain event it is unlikely hydrolysis will occur to any significant extent.
Photolysis is the dominant mechanism by which perfluoro(2-methyl-3-pentanone) is removed from the atmosphere. It has been established previously that photolysis of perfluoro(2-methyl-3-pentanone) in the presence and absence of NOx will lead to the formation of CF3C(O)F in a molar yield of approximately 100%. The atmospheric fate of CF3C(O)F is hydrolysis to give trifluoroacetic acid (TFA). TFA is a ubiquitous naturally occurring component of the hydrosphere and the additional burden associated with perfluoro(2-methyl-3-pentanone) photolysis is not significant. In the present work, we also show that small amounts of PFPrA are also formed as a result of perfluoro(2-methyl-3-pentanone) photolysis. To provide a crude upper limit estimate for the amount of PFPrA that might be expected in precipitation as a result of atmospheric degradation of PFMP we applied the following logic (see SI for details). The production of perfluoro(2-methyl-3-pentanone) by 3M is 100-1000 t year-1 and began in approximately 2003. Given PFMP is used entirely in fire-protection systems that are released by an alarm, it can be considered stored emission potential with releases averaging 1-3% year-1. Combining the upper limit of production (1000 t year-1 ) with a 3% emission factor provides an upper limit of 30 t of perfluoro(2-methyl-3-pentanone) released into the atmosphere each year. Reactions subsequent to the formation of CF3CF2C(O) radicals in air in the presence of excess HO2 give PFPrA in a molar yield of 24%. Using a simple model (see SI), and assuming the photolysis of perfluoro(2-methyl-3-pentanone), Jackson and his coworkers derive an upper limit of 0.6 ng L-1 for the average concentration of PFPrA in precipitation resulting from PFMP oxidation.[1]
Application research
Preparation and thermal responsiveness of microencapsulated fluorinated liquids for automatic fire extinguishing[2]
(1) Under appropriate conditions, perfluoro(2-methyl-3-pentanone) can undergo microencapsulation using UF as a wall material through in-situ polymerization. UF demonstrates a thermal insulation effect, raising the phase-transition temperature of microencapsulated perfluoro (2-methyl-3-pentanone) from 49℃ to 120℃.
(2) DMTP can be microencapsulated via solvent evaporation with PMMA as a wall material under suitable conditions. Moreover,the response temperature for DMTP microencapsulation spans from 88℃ to 95℃.
(3) The encapsulation rate of microcapsules is intricately linked to the preparation method and the selection of core and wallmaterials. In this study, UF@perfluoro(2-methyl-3-pentanone) microcapsules achieved an encapsulation rate of 70 %, while PMMA@DMTP microcapsulesreached approximately 50%.
(4) Fuel containing additional UF@Perfluoro(2-methyl-3-pentanone) and PMMA@DMTP microcapsules could self-extinguish during combustion. When the fluoride solution of the core material attains the threshold temperature, the gas pressure generated in the vaporization-phase change can breach the shell, releasing gas for active fire protection.
(5) In scenarios where the extra UF@perfluoro(2-methyl-3-pentanone) and PMMA@DMTP microcapsules in the fuel fail to achieve self-extinguishing,enhancing the material’s fire-resistant performance is possible by reducing the quantity of fire-extinguishing agents in the extinguishing process. This involves delaying the ignition time of the fuel and diminishing the HRR during combustion.Furthermore, the degree of improvement is strongly correlated with the additional amount, as well as the type of wall and core materials in the microcapsules.
(6) The mechanism of UF@perfluoro(2-methyl-3-pentanone) microcapsules in enhancing the fire-resistant performance of materials incorporates a comprehensive blend of physical and chemical effects. It demonstrates a more effective improvement compared with PMMA@DMTP microcapsules, which operate with a single physical mechanism.
References
[1]Jackson DA, Young CJ, Hurley MD, Wallington TJ, Mabury SA. Atmospheric degradation of perfluoro-2-methyl-3-pentanone: photolysis, hydrolysis and hydration. Environ Sci Technol. 2011;45(19):8030-8036. doi:10.1021/es104362g
[2]Liu H, Zhang T, Zhang M, et al. Preparation and thermal responsiveness of microencapsulated fluorinated liquids for automatic fire extinguishing. Heliyon. 2024;10(5):e27454. Published 2024 Mar 3. doi:10.1016/j.heliyon.2024.e27454
You may like
See also
Lastest Price from Perfluoro(2-methyl-3-pentanone) manufacturers

US $0.00-0.00/kg2025-04-22
- CAS:
- 756-13-8
- Min. Order:
- 5kg
- Purity:
- 99%
- Supply Ability:
- 20MT

US $10.00/KG2025-04-21
- CAS:
- 756-13-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt