Osmium - The World's Rarest And Densest Metal
It is unlikely that "osmium" is the first thought to enter your head upon waking. And that's a damn good thing because if so, this would not bespeak well of your mental wellbeing.
Speaking of mental wellbeing, we chemists are known to be disturbingly short on it, as is evidenced by the fact that osmium somehow migrated from the depths of my twisted subconsciousness to my frontal lobe, hence resulting in this article.
But just cause I'm deeply disturbed does not mean that this isn't an interesting topic. It is. Osmium is rare and has some distinctive properties that make it a fascinating element. It is plopped in the middle of the periodic table and has some mighty strange neighbors, some of which don't really exist (1). But it also has some valuable neighbors - palladium, silver, platinum, and gold (Figure 1).
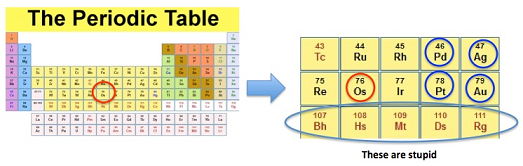
Figure 1. (Left) The periodic table with osmium circled in red. (Right) A closeup of the section of the table where osmium resides. It is found in a group which contains precious metals and weird metals. Osmium is both.

This makes osmium both strange and valuable. In fact, it is so strange that it is the least abundant element in the earth's crust. For every 1 gram of osmium, there are 307,333,333 grams of oxygen, the most abundant element. This scarcity is reflected in the metal's price (per ounce).
Silver - $15
Gold - $1,189
Platinum - $794 (2)
Osmium - $935 (3)
Osmium is also the densest of all metals. Its density of 22.6 g/mL makes it 22.6 times heavier than water. Metal densities are all over the place, with the lightest metals being at the top of the periodic table and the heaviest at the bottom. Here are a few examples (in grams/mL):
Lithium 0.53
Sodium 0.97
Potassium 0.89 (4)
Iron 7.9
Lead 11.3
Mercury 13.5
Gold 19.3
What is it used for?
Because of its chemical inertness, durability, and hardness, osmium is used to make electrical contacts, phonograph needles, and fountain pens. But when it is combined with four atoms of oxygen you get an entirely different beast - osmium tetroxide, which is 50 shades of nasty. It is toxic as hell, but the real danger is blindness, something chemistry blogger Derek Lowe wrote about in 2004:
Breathing a large amount of the vapor can kill a person through irritation of the lungs, but it’s not as bad that way as the better-known agents like phosgene. A bigger problem is the cornea of the eyes, and the reagent is mostly feared for its ability to bring on temporary (and in some cases, permanent) blindness... just being around the solid crystals is enough to get you overexposed to the vapors."
Derek Lowe, In the Pipeline, 2004
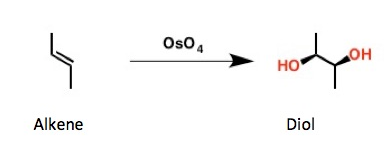
But many organic chemists have used it for one main reason - to covert an alkene to a diol. Osmium tetroxide performs this transformation very well (5). It is also used to stain icky living things on microscope slides. Chemists don't like icky living things.
So now you know everything you've wanted to know about osmium (probably nothing) and more. But at least you'll be less dense.
NOTES:
(1) A bunch of stupid radioactive metals. They don't exist naturally (they are called "synthetic elements"). Instead, they are created by bombarding real elements with high energy particles. And they don't stick around very long. Here are the half-lives for the five in Figure 1. The range is due to the presence of different isotopes.
Bohrium (Bh) - 1-10 minutes
Einsteinium (Es) 40-480 days
Meitnerium (Mt) 0.4-60 seconds
Darmstadtium (Ds) 0.2-14 seconds
Roentgenium (Rg) Milliseconds - minutes
(2) Since when is gold more valuable than platinum???? Since you asked, since 2011. Who knew?
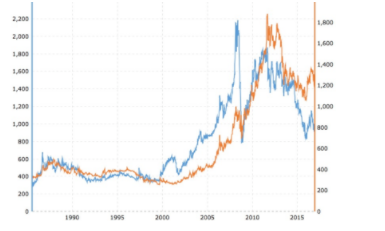
Gold (orange) vs. platinum in USD. Source: Jewelove
(3) Despite its rarity osmium isn't even close to the most expensive naturally occurring metal in the world. That honor goes to rhodium at almost $2,400 per ounce. But einsteinium, a synthetic element, puts this to shame at $27,000,000 per gram ($950,000 per ounce). And it is useless.
(4) If you've ever dropped a chunk of sodium or potassium into a glass of water they float on top because they are less dense than water. They also go spinning around the surface like madmen, catch fire and then blow up. Cool.
(5) Yes. I know that two isomers are formed. I just didn't feel like drawing both of them. Deal.