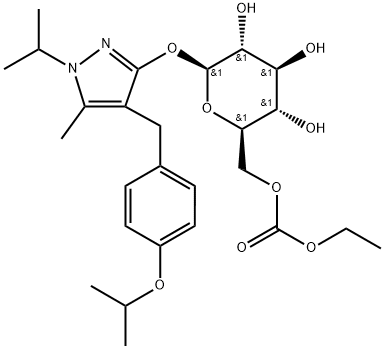
One of the SGLT2 inhibitors: ReMogliflozin etabonate
Description
Remogliflozin etabonate (RE) is the prodrug of remogliflozin, a selective sodium−glucose cotransporter subtype 2 (SGLT2) inhibitor discovered by Kissei Pharmaceutical. RE is the latest addition to the SGLT2 inhibitor class of drugs recently approved in India for managing Type 2 diabetes mellitus (T2DM). RE is a potent and selective inhibitor of SGLT2 with the unique distinction of being administered as a prodrug, existence of active metabolites, and short half-life necessitating twice-daily dosing[1].
SGLT2 inhibitors
Inhibition of SGLT2 lowers blood glucose concentrations by meditating glucose reabsorption from glomerular filtrate. Several SGLT2 inhibitors, including canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin, have been approved globally or are currently under development. The ester prodrug exhibited good absorption and was shown to be rapidly hydrolyzed to the active compound in the gastrointestinal mucosa. The drug has good selectivity for SGLT2 over SGLT1 (Ki = 12.4 and 4520 nM, respectively) but a short half-life (t1/2 = 2−4 h) compared with other SGLT2 inhibitors. In a Phase III clinical trial in India, remogliflozin etabonate (100 mg or 250 mg administered twice daily) demonstrated noninferiority to dapagliflozin (10 mg once daily).
Synthesis method
A patent disclosed by Glenmark Pharmaceuticals reported a synthesis of remogliflozin etabonate originating from 4- (hydroxymethyl)phenol, and this approach is shown below[2]. Phenol 202 was converted to 1,2-dihydro-3Hpyrazol-3-one 204 using a four-step sequence without purification of intermediates. Alkylation of the phenol with isopropyl bromide was followed by chlorination of the benzyl alcohol moiety. The reaction of the resultant benzyl chloride with methyl acetoacetate in the presence of lithium chloride, sodium iodide, and DIPEA gave β-keto ester 203. This intermediate was further treated with hydrazine monohydrate to produce pyrazolone 204, which was recrystallized from water. In an interesting series of operations, regioselective Nisopropylation of the pyrazolone group was achieved via initial O-methylation of 204 followed by subjection to isopropyl bromide and finally, desulfonation with an aqueous base to generate isopropyl pyrazolone 205.
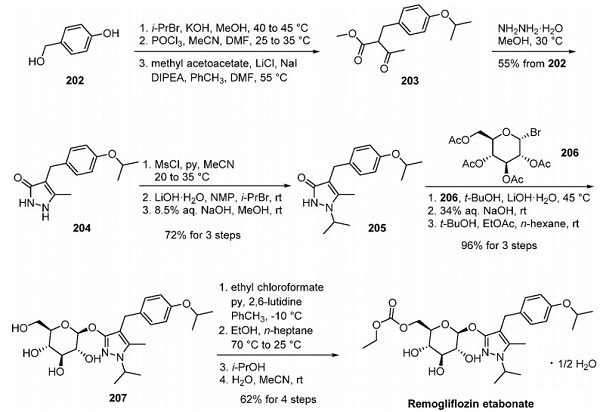
Stirring 205 with Oacetyl-D-glucopyranosyl bromide (206) and lithium hydroxide monohydrate in tert-butanol introduced the glucose ring system in high yield. Deacetylation of the crude product with aqueous hydroxide afforded a remogliflozin-free base (207), which was then purified by crystallization from tert-butanol, ethyl acetate, and n-hexane. Reaction with ethyl chloroformate provided remogliflozin etabonate (XXIII), which was isolated by filtration and crystallized from isopropanol first to give the isopropanol solvate of remogliflozin etabonate. This form was further crystallized from water and acetonitrile to provide remogliflozin etabonate hemihydrate in 62% yield.
References
[1] Viswanathan Mohan. “Remogliflozin Etabonate in the Treatment of Type 2 Diabetes: Design, Development, and Place in Therapy.” Drug Design, Development and Therapy (2020): 2487–2501.
[2] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.