How to synthesize Sotagliflozin?
Description
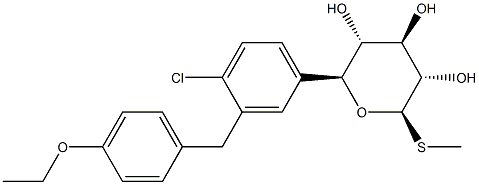
Sotagliflozin (LX-4211) is a dual sodium-glucose co-transporter-2 and 1 (SGLT2/1) inhibitor for the treatment of both type 1 (T1D) and type 2 diabetes (T2D)[1–2].
Mechanism of action
Sotagliflozin inhibits renal sodium-glucose co-transporter 2 (determining significant excretion of glucose in the urine, in the same way as other, already available SGLT-2 selective inhibitors) and intestinal SGLT-1, delaying glucose absorption and therefore reducing post-prandial glucose. have shown that sotagliflozin (as monotherapy or add-on therapy to other anti-hyperglycemic agents) improves glycated hemoglobin in adults with T2D, with beneficial effects on body weight and blood pressure[3].
Synthesis method
A process reportedly capable of delivering thousands of kilograms of sotagliflozin for clinical studies was reported by Lexicon Pharmaceuticals in their 2010 patent[4]. The convergent route hinged upon the union of aryl iodide fragment 211 with furanose subunit 216 through a ketone synthesis strategy.
Synthesis of Aryl Iodide Fragment 211
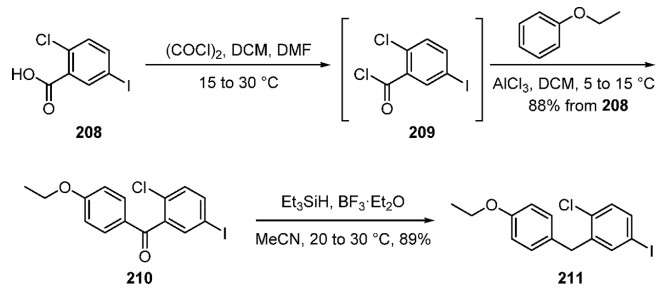
Fragment 211 was accessed starting from benzoic acid 208. Activation of the carboxylic acid to give acid chloride 209 followed by Friedel−Crafts acylation with ethyl phenyl ether furnished arene 210 in good yield. The regioselectivity of the Friedel−Crafts acylation was high (∼100:1), provided that the water content of the DCM was low (0.01% w/w). Although the largest-scale report (450 kg scale) of the triethylsilyl hydride reduction of ketone 211 was conducted in high yield and purity, alternative conditions were also developed using NaBH4 and AlCl3 (250 kg scale) in attempts to reduce the cost of the process.
Synthesis of Sotagliflozin
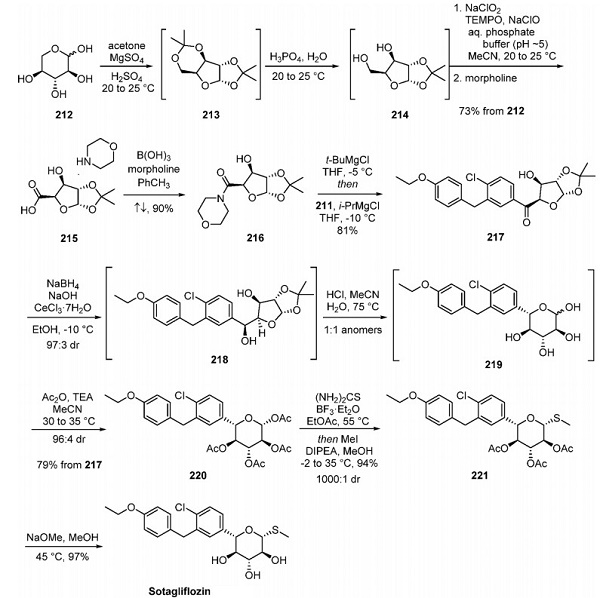
The core sugar moiety of the API was ultimately derived from L-(−)-xylose (212). Global acetonide protection of 212, involving rearrangement of the pyranose to the furanose, gave bis(acetonide) 213. Selective deprotection to give 214 occurred under acidic conditions with careful control of the pH (1.5−2.0), temperature, and duration to minimize over-hydrolysis. Subsequent selective oxidation of the primary alcohol 214 was performed using NaClO2 with catalytic TEMPO and NaClO and controlled by slowly adding reagents. The resulting carboxylic acid was then isolated as morpholine salt 215 as a crystalline intermediate in 73% overall yield from 212. This was particularly advantageous because morpholine was required in the subsequent amidation step, and the formation of the salt avoided the isolation of the free acid, which was prone to self-deprotection. The formation of morpholine amide 216 was performed with catalytic B(OH)3 under Dean−Stark conditions. Treatment of amide 216 with tert-butylmagnesium chloride led to deprotonation of the secondary alcohol, providing an intermediate that could be reacted with the Grignard reagent of fragment 211 (generated by treatment with isopropylmagnesium chloride) to afford ketone 217 without any loss of stereointegrity. Diastereoselective reduction of the carbonyl using Luche conditions gave 218 with a high diastereomeric ratio, and the crude product solution could be taken forward without further workup. Acetonide deprotection and concomitant ring expansion of 218 by subjection to acidic conditions in warm acetonitrile gave 219 as a 1:1 mixture of anomers. Subsequent dynamic kinetic resolution (DKR) via stereoselective acetylation gave 220 with high dr and a good overall yield from 217. This compound was then reacted with thiourea and boron trifluoride diethyl etherate to install the thiol, which was subsequently methylated with iodomethane to give 221 with excellent diastereomeric excess in high yield. Finally, global deprotection of the acetate groups was achieved with sodium methoxide to furnish sotagliflozin.
References
[1] Georgios Chatzopoulos, Konstantinos Tziomalos. “An up-to-date evaluation of sotagliflozin for the treatment of type 1 diabetes.” Expert Opinion on Pharmacotherapy 21 15 (2020): 1799–1803.
[2] Deeks, E. “Sotagliflozin: A Review in Type 1 Diabetes.” Drugs 79 1 (2019): 1977–1987.
[3] Chiara Maria Assunta Cefalo. “Sotagliflozin, the first dual SGLT inhibitor: current outlook and perspectives.” Cardiovascular Diabetology 18 1 (2019): 20.
[4] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
See also
Lastest Price from LX-4211 manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 1018899-04-1
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $999.00-900.00/kg2025-04-21
- CAS:
- 1018899-04-1
- Min. Order:
- 0.0010000000474974513kg
- Purity:
- 99%
- Supply Ability:
- 5000