Nilotinib hydrochloride monohydrate: pharmacodynamic, pharmacokinetic and tolerability
General Description
Nilotinib hydrochloride monohydrate is a promising targeted therapy for chronic myeloid leukemia (CML) that inhibits the tyrosine kinase activity of the BCR-ABL protein. It demonstrates higher binding affinity and selectivity for ABL kinase compared to imatinib and is effective in patients with a range of BCR-ABL mutations associated with imatinib resistance. Nilotinib hydrochloride monohydrate's pharmacokinetics are complex, with peak serum concentrations and AUC values increasing with once-daily doses of 50-400 mg. The recommended dosage of 400 mg twice daily achieves a mean peak serum concentration of 3.6 μmol/L after approximately 3 hours. Nilotinib hydrochloride monohydrate's tolerability profile is generally manageable, with commonly reported adverse events including rash, nausea, pruritus, headache, and fatigue. Hematologic adverse events were observed in a significant proportion of patients, but serious adverse events were infrequent.

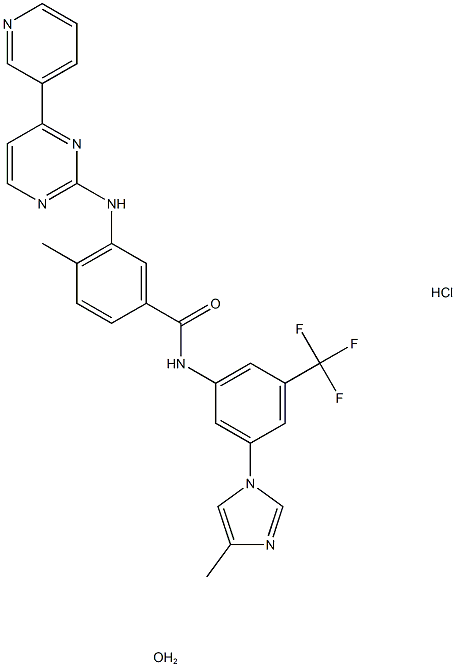
Figure 1. Nilotinib hydrochloride monohydrate
Pharmacodynamic
Nilotinib hydrochloride monohydrate is an aminopyrimidine derivative that inhibits the tyrosine kinase activity of the chimeric protein BCR-ABL, which causes chronic myeloid leukemia (CML). Nilotinib acts as a competitive inhibitor at the ATP-binding site of the BCR-ABL protein and has a higher binding affinity and selectivity for ABL kinase compared to imatinib, another CML treatment. Once bound to the ATP-binding site, nilotinib inhibits tyrosine phosphorylation of proteins involved in BCR-ABL-mediated intracellular signal transduction. The inhibitory activity of nilotinib is markedly higher than that of imatinib in both imatinib-sensitive and -resistant CML cell lines. Nilotinib hydrochloride monohydrate demonstrated inhibitory activity in 32 of 33 imatinib-resistant CML cell lines expressing mutant BCR-ABL kinases, except for T3151. In clinical trials, nilotinib was effective in patients with a range of BCR-ABL mutations associated with imatinib resistance, other than T3151, as well as in patients with BCR-ABL-independent resistance. Overall, nilotinib hydrochloride monohydrate is a promising targeted therapy for CML. 1
Pharmacokinetic
Nilotinib hydrochloride monohydrate exhibits complex pharmacokinetics, as demonstrated by various clinical trials and studies. When administered orally in the phase I trial, trough serum concentrations consistently surpassed the IC50 for cellular phosphorylation of BCR-ABL, even in the presence of BCR-ABL mutants. At steady state, peak serum concentrations and AUC values increased with once-daily doses of 50-400 mg but plateaued at higher doses due to potential gastrointestinal absorption saturation. Additionally, drug exposure was 35% higher with 400 mg twice daily compared to 800 mg once daily, and the recommended dosage of 400 mg twice daily achieved a mean peak serum concentration of 3.6 μmol/L after approximately 3 hours. Nilotinib hydrochloride monohydrate's bioavailability is influenced by food, showing an 82% increase when taken with a high-fat meal. Metabolically, oxidation and hydroxylation are primary pathways, with the main metabolite being the carboxylic acid derivative, though it does not significantly contribute to the drug's activity. Nilotinib demonstrates an elimination half-life of approximately 17 hours, with over 90% of the dose eliminated in the feces within 7 days. Furthermore, concomitant administration with inhibitors or substrates of CYP3A4 can affect Nilotinib hydrochloride monohydrate's systemic exposure, indicating its potential role as a weak inhibitor of CYP3A4. 2
Tolerability
The tolerability of nilotinib hydrochloride monohydrate has been extensively studied in various clinical trials, particularly in patients with imatinib-resistant or -intolerant chronic myeloid leukemia (CML). In a phase II trial involving 280 such patients, receiving either 400 mg or 600 mg of nilotinib twice daily, non-hematologic adverse events such as rash, nausea, pruritus, headache, and fatigue were commonly reported, yet generally of mild to moderate severity. Moreover, hematologic adverse events, including grade 3 or 4 neutropenia and thrombocytopenia, were observed in a significant proportion of patients. Overall, dosage interruptions and reductions were required in a minority of patients, while other laboratory abnormalities were generally mild to moderate in severity. Importantly, the occurrence of serious adverse events such as fluid retention, QTc interval prolongation, and Torsades de pointes was infrequent. These findings suggest that Nilotinib hydrochloride monohydrate demonstrated a tolerable profile in these patients, with manageable adverse events that were largely consistent across different phases of CML and various clinical trials. 3
Reference
1. Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant BcrAbl. Cancer Cell, 2005, 7(2): 129-141.
2. Plosker GL, Robinson DM. Nilotinib. Drugs, 2008, 68(4): 449-459.
3. Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective Bcr-Abl tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosomepositive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood, 2007, 110(10): 3540-3546.
You may like
Related articles And Qustion
See also
Lastest Price from Nilotinib Hydrochloride Monohydrate manufacturers

US $0.00/g2025-01-13
- CAS:
- 923288-90-8
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 100kg/Month

US $0.00/g2025-01-13
- CAS:
- 923288-90-8
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 100kg/Month