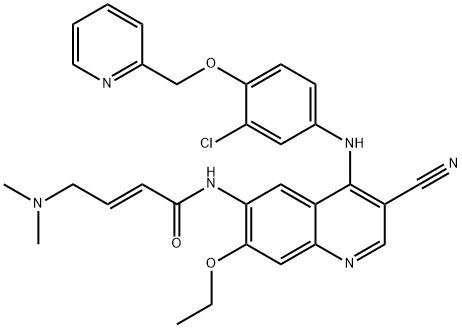
Neratinib- pharmacology
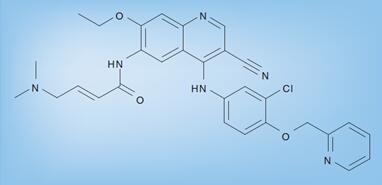
Neratinib is an oral pan HER inhibitor that irreversibly inhibits the tyrosine kinase activity of epidermal growth factor receptors, EGFR (or HER1), HER2, and HER4, which leads to reduced phosphorylation and activation of downstream signaling pathways. Neratinib targets tyrosine kinase activity at the intracellular domain of the HER receptors (EGFR, HER2, and HER4) and trastuzumab binds to the extracellular domain of the HER2 receptor[1].
The initial phase I trial of neratinib showed the maximum tolerated dose (MTD) to be 240 mg/d with the most common dose limiting toxicities being diarrhea (84%), nausea (55%), asthenia (45%), anorexia (31%), vomiting (29%), chills (12%), and rash (10%).[2] Neratinib in combination with weekly paclitaxel and trastuzumab was tested in a phase I trial (National Surgical Adjuvant Breast and Bowel Project FB8 [NSABP FB8]) with the MTD for neratinib determined as 200 mg/d. Of the 21 patients enrolled in this study 38% of the patients had a complete or partial response with a clinical benefit of complete/partial response and stable disease seen in 52% patients.[3]
Neratinib was tested in a phase II trial as monotherapy in 2 cohorts of patients with advanced HER2-positive breast cancer those with and those without previous trastuzumab treatment. Sixteen-week progression-free survival (PFS) rates were 59% for patients with previous trastuzumab treatment and 78% for patients with no previous trastuzumab treatment with a median PFS of 22.3 and 39.6 weeks, respectively. Objective response rates were 24% among patients with previous trastuzumab treatment and 56% in the trastuzumab-naive cohort.[4]
The predominant target organs of toxicity for neratinib in rats and dogs following repeat dosing were the liver, lymph nodes, skin, gastrointestinal system, and mammary gland in males. Toxicity findings were consistent with the clinical adverse reactions reported in clinical trials, the majority of which (e.g., gastrointestinal and skin) are likely related to the pharmacologic inhibition of EGFR, HER2, or HER4. In a rat model of neratinibinduced diarrhea, budesonide was the most effective intervention tested against neratinib-induced diarrhea, which may guide future studies aimed at testing mitigation strategies for neratinibinduced diarrhea[5].
Neratinib and its metabolites were not genotoxic. Administration of neratinib to pregnant rabbits during organogenesis resulted in abortions, embryo-fetal death, and fetal abnormalities at maternal exposures (AUC) approximately 0.2 times exposures in patients at the recommended dose. Oral administration of neratinib to pregnant rats from gestation day 7 until lactation day 20 resulted in effects on long-term memory in male offspring at maternal doses less than the maximum recommended clinical dose on a mg/m2 basis. Neratinib was not carcinogenic in a 26-week carcinogenicity study in rasH2 transgenic mice[6].
On July 17, 2017, the FDA approved neratinib (NERLYNX; Puma Biotechnology, Inc.) for the extended adjuvant treatment of adult patients with early-stage HER2-overexpressed/ amplified breast cancer, to follow adjuvant trastuzumab-based therapy.
References
1.SlamonD,EiermannW,RobertN,Pienkowski T,MartinM,PressM, etal.Adjuvant trastuzumab in HER-2 positive breast cancer[J]. N Engl J Med 2011;365:1273–83.
2.Wong KK, Fracasso PM, Bukowski RM, et al. HKI-272, an irreversible pan ErbB receptor tyrosine kinase inhibitor: preliminary phase 1 results in patients with solid tumors[J]. J Clin Oncol, 2006, 24(18S): abstract 3018.
3.Jankowitz C, Abraham J, Tan A, et al. Safety and efficacy of neratinib in combination with weekly paclitaxel and trastuzumab in women with metastatic HER2-positive breast cancer: an NSABP Foundation Research Program phase I study[J]. Cancer Chemother Pharmacol. 2013, 72:1205-12.
4.Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer[J]. J Clin Oncol. 2010, 28:1301-7.
5.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer[J]. N Engl J Med, 2005, 353:1659–72
You may like
Lastest Price from Neratinib manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 698387-09-6
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS
US $0.00-0.00/kg2025-04-04
- CAS:
- 698387-09-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton