2-Amino-1,3-propanediol: natural occurrence, applications and biological synthesis
General Description
2-Amino-1,3-propanediol is involved in the biosynthesis of toxins in plants and plant pathogens, such as helminthosporoside and rhizobitoxine, catalyzed by specific enzymes. It has diverse applications in medicine and the chemical industry, including fungicides, sphingosines, anti-cancer drugs, and contrast agents for medical imaging. 2-Amino-1,3-propanediol and its derivatives can be synthesized biochemically or through biotechnological approaches, such as using mutants of Pichia ciferri to produce target compounds. However, in vitro derivatization using wax ester synthase/acyl-CoA:diacylglycerol acyltransferase from A. baylyi ADP1 was unsuccessful.

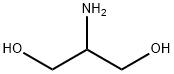
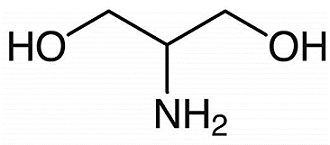
Figure 1. 2-Amino-1,3-propanediol
Natural occurrence
2-Amino-1,3-propanediol plays a role in the biosynthesis of toxins in plants. It is involved in the production of helminthosporoside by the pathogenic fungus Helminthosporium sacchari in sugarcane. The enzyme responsible for 2-amino-1,3-propanediol synthesis has been studied and it has a Km value of 0.1 to 1 mM for serinol. The amino acids glutamine, glutamic acid, and aspartic acid can serve as donors for the transaminase reaction that utilizes serinol. 2-Amino-1,3-propanediol is also an intermediate in the biosynthesis of rhizobitoxine in plant pathogens Burkholderia andropogonis and Bradyrhizobium japonicum, as well as Bradyrhizobium elkanii, which is a symbiont of legumes. Rhizobitoxine inhibits ethylene biosynthesis and enhances nodulation and competitiveness in plants. The gene and protein responsible for the transamination reaction in rhizobitoxine synthesis have not been identified. Several studies have shown that the RtxA protein has a role in serinol accumulation and catalyzes the conversion from dihydroxyacetone phosphate to serinol phosphate, and then to serinol. The preferred amino donors for this transamination reaction are glutamic acid, alanine, and aspartic acid. Mutations in different regions of the RtxA protein affect serinol accumulation and dihydrorhizobitoxine synthesis. In summary, 2-Amino-1,3-propanediol is involved in the biosynthesis of toxins in sugarcane and plant pathogens. It plays a role in the production of helminthosporoside and rhizobitoxine, and its synthesis is catalyzed by specific enzymes whose genes and proteins are not fully characterized. 1
Applications
2-Amino-1,3-propanediol, along with its derivatives, has a wide range of applications in various industries. In the field of medicine, long chain α,ω-aminoalcohols derived from serinol are used as fungicides. Amino acid derived amino alcohols serve as important intermediates for producing enantiomerically pure substances. N-acetyl-1,3-amino alcohols derived from serinol are used in the synthesis of sphingosines, which have dermatological and pharmaceutical applications. 2-Amino-1,3-propanediol also plays a significant role in the development of anti-cancer drugs. Synthetic N-acylated serinols have been shown to increase apoptosis, making them potential candidates for cancer treatment. Additionally, serinol is used in the production of fingolimod, an oral drug used in multiple sclerosis treatment. In the chemical industry, serinol and its derivatives are utilized in the synthesis of non-ionic X-ray contrast agents like iopamidol. These agents are commonly used in angiography procedures for cardiovascular imaging. Furthermore, serinol serves as a precursor for pain treatment drugs. By attaching alkyl chains to the C2 atom of serinol, drugs can be developed to alleviate pain. Overall, 2-Amino-1,3-propanediol has diverse applications in medicine and the chemical industry, ranging from fungicides and dermatological products to anti-cancer drugs and contrast agents for medical imaging. 2
Biological synthesis
The biosynthesis of 2-amino-1,3-propanediol and its derivatives has been studied through various biological approaches. Biotechnological production of serinol derivatives such as sphingosine, dihydrosphingosine, and phytosphingosine has been established using mutants of Pichia ciferri. These strains can produce up to 0.8 g/l of the target compounds under batch culture conditions. 2-Amino-1,3-propanediol can be synthesized biochemically by amino alcohol dehydrogenases (AMDH). An NAD+/NADH-dependent AMDH from Streptomyces virginiae was isolated and shown to catalyze the dehydrogenation of serinol, producing DHA, ammonium, and NADH. Efforts were made to convert serinol into its acylester form, but in vitro derivatization using wax ester synthase/acyl-CoA:diacylglycerol acyltransferase (WS/DGAT) from A. baylyi ADP1 was unsuccessful. 3
Reference
1. Babczinski P, Matern U, Strobel GA. Serinol phosphate as an intermediate in serinol formation in sugarcane. Plant Physiol, 1978, 61:46–49.
2. Nicholas GM, Li RH, MacMillan JB, Molinski TF. Antifungal activity of bifunctional sphingolipids. Intramolecular synergism within long-chain alpha, omega-bis-aminoalcohols Bioorg Med Chem Lett, 2002, 12:2159–2162.
3. Nakazawa H, Enei H, Kubota K, Okumura S. Biological method of producing serine and serinol derivatives. 1975, US patent 3871958.
You may like
Related articles And Qustion
See also
Lastest Price from 2-Amino-1,3-propanediol manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 534-03-2
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 1000kgs

US $10.00/kg2025-04-21
- CAS:
- 534-03-2
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100 TON