2-Amino-1,3-propanediol: Overview, Biological Functions and its Derivatives
General Description
2-Amino-1,3-propanediol is a stable and prochiral compound with a molecular formula of C3H9NO2. It serves as a crucial precursor in the biosynthesis of phospholipids, contributing to cell membrane integrity and fluidity. Additionally, it is involved in neurotransmitter synthesis and sphingolipid metabolism, impacting neurological functions and cellular signaling. Natural derivatives of 2-Amino-1,3-propanediol, such as sphingosines and ceramides, act as central second messengers in cellular processes and have been identified in marine organisms and yeasts. Overall, 2-Amino-1,3-propanediol and its derivatives play significant roles in biological functions and have widespread applications in chemical and biological contexts.

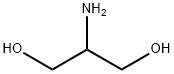
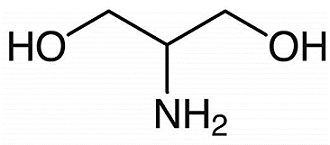
Figure 1. 2-Amino-1,3-propanediol
Overview
2-Amino-1,3-propanediol, also known as serinol, is a stable and prochiral compound belonging to the group of amino alcohols. With a molecular formula of C3H9NO2, it bears structural similarity to the amino acid serine. It is highly stable, corrosive, and hygroscopic, exhibiting excellent solubility in water. With a molecular weight of 91.11 g/mol, it melts at 52 to 56 °C and boils at 115 to 116 °C. The term "serinol" encompasses a range of C-substituted commercial analogs, and like many amino acids, 2-Amino-1,3-propanediol and its derivatives are commonly used as intermediates in various chemical applications. In biological systems, 2-Amino-1,3-propanediol derivatives serve as central second messengers in many eukaryotic organisms. Additionally, in certain prokaryotes, 2-Amino-1,3-propanediol functions as an intermediate in toxin synthesis. Due to its versatile chemical properties and biological significance, 2-Amino-1,3-propanediol and its derivatives have found widespread applications in both chemical and biological contexts. 1
Biological functions
2-Amino-1,3-propanediol, also known as serinol, is a crucial compound with significant biological functions in living organisms. This amino alcohol plays a vital role in various biochemical processes due to its unique structure and properties. One of the key biological functions of 2-Amino-1,3-propanediol is its involvement in the biosynthesis of phospholipids, which are essential components of cell membranes. As a precursor in the production of phosphatidylserine, serinol contributes to maintaining the structural integrity and fluidity of cell membranes, enabling proper cell signaling and transport of molecules across the membrane. Moreover, 2-Amino-1,3-propanediol serves as a building block for the synthesis of certain neurotransmitters in the brain, such as serotonin. Serotonin plays a crucial role in regulating mood, sleep, and appetite, highlighting the importance of serinol in neurological functions and mental well-being. Additionally, serinol is involved in the metabolism of sphingolipids, which are another class of lipids important for cell signaling and cell-cell interactions. By participating in sphingolipid metabolism, 2-Amino-1,3-propanediol contributes to cellular processes such as growth, differentiation, and apoptosis. In conclusion, 2-Amino-1,3-propanediol plays a critical role in the biosynthesis of essential biomolecules, cell membrane structure, neurotransmitter synthesis, and cellular signaling pathways, highlighting its significance in various biological functions within living organisms. 1
2-Amino-1,3-propanediol derivatives
2-Amino-1,3-propanediol has various derivatives that occur naturally in living organisms. One important class of derivatives is the acylated serinols, specifically sphingosines. Sphingosine, along with its N-acylated derivative ceramide, serves as a central second messenger in eukaryotes, regulating cell growth, endocytosis, stress response, and apoptosis. These 2-Amino-1,3-propanediol derivatives have been found in marine sponges, such as Stelletta inconspicua. Inconspicamide, an N-acylated serinol (N-palmitoyl-2-amino-1,3-propanediol), has been extracted from this sponge species. The synthesis of sphingosine and ceramide is well-described in yeasts, particularly Saccharomyces cerevisiae. The process involves the condensation of serine and palmitoyl-coenzyme A (CoA) to form 3-ketodihydrospingosine catalyzed by a serine palmitoyltransferase. This compound is further reduced to dihydrosphingosine by a 3-ketosphinganine reductase. Ceramide is then synthesized by the addition of a second palmitoyl moiety from palmitoyl-CoA, facilitated by a ceramide synthase. Additional modifications can occur through processes like hydroxylation. One yeast species of industrial interest, Pichia ciferri (formerly Hansenula ciferri), secretes tetra-acetyl-phytosphingosine (TAPS) as a crystalline form into the medium. TAPS can be easily purified from the medium. In summary, natural derivatives of 2-Amino-1,3-propanediol, such as sphingosines and ceramides, play important roles as second messengers in cellular processes and have been identified in marine organisms and yeasts. 2
Reference
1. Andreeßen B, Steinbüchel A. Serinol: small molecule - big impact. AMB Express. 2011;1(1):12.
2. Uchida Y, Nardo AD, Collins V, Elias PM, Holleran WM. De novo ceramide synthesis participates in the ultraviolet B irradiation-induced apoptosis in undifferentiated cultured human keratinocytes. J Invest Dermatol. 2003;120(4):662-669.
Related articles And Qustion
Lastest Price from 2-Amino-1,3-propanediol manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 534-03-2
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 1000kgs

US $10.00/kg2025-04-21
- CAS:
- 534-03-2
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100 TON