Mezlocillin, Azlocillin: Antimicrobial Activity, Susceptibility, Administration and Dosage, Clinical Uses etc.
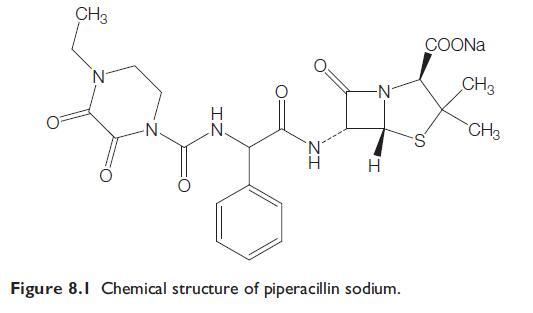
Mezlocillin and Azlocillin are described together because they are often referred to as ‘‘newer antipseudomonal penicillins.’’ They are all vulnerable to the action of many beta-lactamases, but most of them are considerably more active in vitro than carbenicillin and ticarcillin against Pseudomonas aeruginosa, other Gram-negative bacteria, and some Gram-positive bacteria. These drugs are acylamino penicillins. Because mezlocillin and azlocillin each contain a ureido (-N-CO-N-) group, they are called ureido-penicillins (Slack, 1981; Selwyn, 1982). Piperacillin is now the key drug in this group that is commonly available, either alone or in the fixed combination of piperacillintazobactam.
a. Mezlocillin
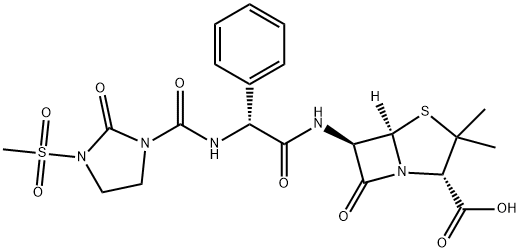
This has the chemical formula D-alpha (2-oxo-3-mesyl-imidazolidinyl)- carbonyl amino-benzyl-penicillin. It is an ureido-penicillin and resembles an alpha-amino-substituted ampicillin (Bodey and Pan, 1977; Wise and Andrews, 1982). Mezlocillin alone is available for clinical use as Mezlint. It is now rarely used clinically, but has been replaced in a small number of regions by a fixed combination of mezlocillin and sulbactam, a beta-lactamase inhibitor.
b. Azlocillin
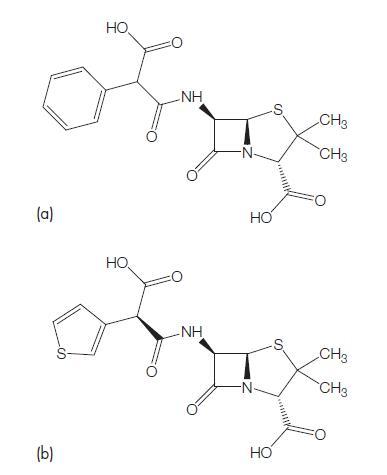
Azlocillin is another ureido-substituted penicillin that has the chemical formula 6D-2-(2-oxoimidazolidine-1-carboxamido-2-phenylacetamido)- penicillanic acid (Stewart and Bodey, 1977). Azlocillin can also be considered as an alpha-amino-substituted ampicillin (Wise et al., 1982). Azlocillin is now not available for clinical use.
ANTIMICROBIAL ACTIVITY
a. Routine susceptibility
The antibacterial spectra of these compounds are similar to those of carbenicillin and ticarcillin, but there are differences between their degree of activity against various bacterial species. All of these antibiotics have lost much of their activity owing to emergence of resistance. To some extent, that problem has been reduced for mezlocillin and piperacillin, which are available in fixed combinations with sulbactam and tazobactam, respectively.
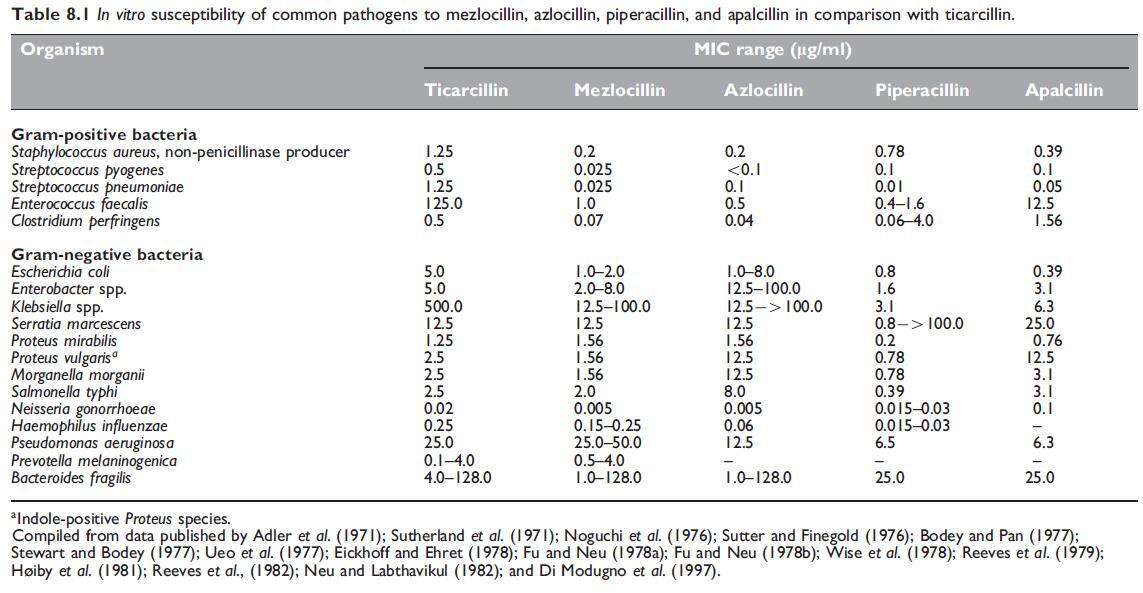
The comparative in vitro susceptibility data for these agents against common pathogens are shown in Table 8.1.
Mezlocillin
Mezlocillin is more active than carbenicillin and ticarcillin against most Eschericia coli, Enterobacter spp., Proteus vulgaris, P. rettgeri, and Morganella morganii strains. It is also more active than ticarcillin against Klebsiella spp.; when introduced, mezlocillin inhibited more than 50% of strains at clinically achievable concentrations (Fu and Neu, 1978b;Wise and Andrews, 1982) (see Table 8.1). Activity of mezlocillin was about the same as that of ticarcillin against Serratia marcescens and P. aeruginosa (Parry and Folta, 1983). As with azlocillin (see below under Azlocillin), the activity of mezlocillin against P. aeruginosa was markedly inoculum dependent and MBCs are much higher than MICs, suggesting that each culture contains some highly mezlocillin-resistant P. aeruginosa strains (Bodey and Pan, 1977; Greenwood and Eley, 1982; Korvick et al., 1987).
As mezlocillin is vulnerable to many beta-lactamases of Gram-negative bacteria, many isolates, especially hospital-associated strains of these bacteria may be highly mezlocillin resistant (Ellis et al., 1979;White et al., 1979). Mezlocillin and other acylamino penicillin derivatives are not resistant to TEM beta-lactamases, which are the most frequent plasmidmediated beta-lactamases among resistant Enterobacteriaceae (Eliopoulos and Moellering, 1982). However, some E. coli isolates which produce TEM-1 beta-lactamase remain relatively mezlocillin sensitive, whereas they are resistant to ampicillin (Livermore et al., 1986).
Mezlocillin is active against Haemophilus influenzae, its activity exceeding that of ampicillin (Sanders, 1982a). Meningococci and gonococci are quite sensitive. Strains of Neisseria gonorrhoeae highly susceptible to penicillin G are equally sensitive to mezlocillin. Strains with intermediate susceptibility to penicillin G (MICs 0.125–0.5 mg/ ml) are much more sensitive to mezlocillin (MICs 0.0004–0.125 mg/ ml).
Mezlocillin is highly active against Gram-positive bacteria, such as Streptococcus pyogenes, group B streptococci, S. pneumoniae, and S. viridans, but penicillin G and ampicillin are more active against these organisms. Mezlocillin is slightly less active than ampicillin, but equally active as penicillin G against Enterococcus faecalis (Sanders, 1981). Some authors have found that mezlocillin, unlike penicillin G and ampicillin, has identical MICs and MBCs against this organism (Moody et al., 1984).
Azlocillin
The main advantage of this drug is that its activity against P. aeruginosa is somewhat superior to that of carbenicillin, ticarcillin, and mezlocillin (see Table 8.1). It is also active against many carbenicillin-resistant strains of this organism (Stewart and Bodey, 1977; Coppens and Klastersky, 1979; Parry, 1983). Azlocillin-resistant P. aeruginosa strains were uncommon; in 24 UK hospitals only 3.9% of strains were resistant with MICs greater than 32 mg/ml (Williams et al., 1984).
The superiority of azlocillin over other antipseudomonal penicillins has been disputed. When conventional MIC tests and small inocula are used, azlocillin has lower MICs than the other drugs against P. aeruginosa (Table 8.1).
Compared with mezlocillin, azlocillin had the same activity against Bacteroides fragilis, but it is somewhat less active against most other Gram-negative bacilli (Table 8.1) (Fu and Neu, 1978b; Ellis et al., 1979).
Apalcillin
Pseudomonas aeruginosa is as sensitive to this drug as it is to piperacillin (see Table 8.1), although some authors have found apalcillin to be more active than piperacillin against this organism (Barry et al., 1984; Barry et al., 1985). Against most Enterobacteriaceae, such as E. coli and Citrobacter, Klebsiella, Enterobacter, Proteus, and Providencia spp., apalcillin is similar to mezlocillin and piperacillin. It is somewhat less inhibitory than these two drugs against Serratia spp., but B. fragilis is equally sensitive to apalcillin and piperacillin (Table 8.1) (Noguchi et al., 1976; Neu and Labthavikul, 1982; Wexler et al., 1984).
b. Emerging resistance and cross-resistance
Similar to mezlocillin and azlocillin, piperacillin is susceptible to betalactamases, so that many Gram-negative bacteria with acquired resistance to ampicillin or carbenicillin are also piperacillin resistant (Verbist, 1978; Reeves et al., 1982; Dornbusch et al., 1990).
With P. aeruginosa, intrinsic resistance to piperacillin due to changes in penicillin-binding proteins (PBPs), particularly PBP3, has emerged in vivo during piperacillin treatment of P. aeruginosa infections in cystic fibrosis patients (Godfrey et al., 1981). In an in vitro study, piperacillinresistant variants were detected in each of ten strains of P. aeruginosa. Some investigators have shown that quinolone resistance among P. aeruginosa isolates is not associated with piperacillin cross-resistance (Fujita et al., 1992; Radberg et al., 1990; Wu et al., 1999). However, an association between piperacillin and levofloxacin resistance exists when quinolone-resistant isolates are examined for efflux overexpression (Kriengkauykiat et al., 2005).
Mezlocillin (and related penicillins), combined with some betalactamase- resistant cephalosporins, may be antagonistic against certain Gram-negative bacilli. When a mezlocillin–cefoxitin combination was tested against B. fragilis, synergism was observed in 10/20 strains, but there was no antagonism (Bansal and Thadepalli, 1983). In studies in which mezlocillin was combined with either cefoxitin, cefotaxime, cefoperazone, or moxalactam, and tested against Enterobacteriaceae and P. aeruginosa, the most common result was indifference, but antagonism occurred occasionally with mezlocillin– cefoxitin (Neu and Labthavikul, 1982b).
A combination of azlocillin with a beta-lactamase-resistant cephalosporin, such as cefoxitin or cefotaxime, may provide a broad initial cover for the treatment of serious undiagnosed infections; antagonism has not been demonstrated (Ellis et al., 1979; Fass, 1982a). Because beta-lactamase-stable cephalosporins may act as enzyme inducers, it is probably best to avoid using azlocillin with such betalactam antibiotics to treat infections caused by some Gram-negative bacilli, such as Enterobacter, Serratia, and Pseudomonas spp. A combination of piperacillin with an aminoglycoside, such as gentamicin, tobramycin, or amikacin, is synergistic in vitro against many strains of Enterobacteriaceae and P. aeruginosa (Kurtz et al., 1981; Fass 1982b; McAllister, 1982). The combination of piperacillin and amikacin is also more effective than piperacillin–pefloxacin in preventing the emergence of resistant mutants (Boisivon et al., 1988). A combination of piperacillin–gatifloxacin is synergistic in vitro against P. aeruginosa, whereas piperacillin–ciprofloxacin is additive (Dawis et al., 2003; Hyatt et al., 1995).
MECHANISM OF DRUG ACTION
The mode of action of these penicillins on bacteria is similar to that of penicillin G. Their increased activity against P. aeruginosa and other Gram-negative bacilli is mainly due to an ability to pass through the various layers of the cell envelope to reach their target PBPs. Mezlocillin, azlocillin, piperacillin, and apalcillin have an increased affinity for PBP3, and a lesser affinity for PBP1 and 2 of both E. coli and P. aeruginosa. PBP3 is an enzyme, septal murein synthetase, which is responsible for septum formation during bacterial growth and cell division.
MODE OF DRUG ADMINISTRATION AND DOSAGE
a. Adults
Mezlocillin
The usual dosage for adults is 200–450 mg/kg body weight per day, given i.m. or more commonly i.v., in six divided doses. Each dose can be injected directly into the i.v. tubing, but it is preferable to give it as an infusion over 15–30 minutes. A common adult dosage for serious infections is 3 g i.v. 4-hourly or 4 g 6-hourly (Pancoast et al., 1979; Neu, 1982; Collaizzi et al., 1986b).
Azlocillin
This drug was given in a dosage of 100–300 mg/kg body weight per day, or occasionally for severe infections 450 mg/kg/day. It was usually administered in four or six divided doses; each dose given i.v. by a rapid injection or as a 15- to 30-minute infusion. In adults, a dosage varying from 1 to 5 g i.v. 6-hourly was used depending on the severity of the infection (Ellis et al., 1979; Eykyn, 1982; Levy et al., 1982). The usual adult dosage for severe infection was either 4 g every 6 hours or 5 g every 8 hours (Lander et al., 1989).
PHARMACOKINETICS AND PHARMACODYNAMICS
a. Bioavailability
None of these penicillins is absorbed from the gastrointestinal tract and they must be given parenterally (i.v. or i.m.). The half-life of mezlocillin varies depending on the dose and is about 1 hour at a dose of 3 g and increases to about 1.2 hours if the dose is 5 g (Bergan, 1978). The serum protein binding of these drugs depends on their serum concentration. At concentrations of 200 mg/ml, mezlocillin is 27% and azlocillin 30% protein bound. For piperacillin, the mean protein binding is 16% at concentrations in the range 200–300 mg/ml (Bergan, 1981). Similarly, the pharmacokinetics of azlocillin changes when the dose is increased.
b. Drug distribution
Mezlocillin
After i.v. administration of a 3-g dose of mezlocillin given over a 15- minute period, the mean Cmax at the end of the infusion is 269 mg/ml. Thereafter, the serum level falls, and at 6 hours it is less than 10 mg/ml. Its half-life (66 minutes) is similar to that of ampicillin and carbenicillin. When 3 g of mezlocillin is given i.v. every 4 hours as a 2-hour infusion, the peak serum level just after the infusion is over 100 mg/ml, and levels are maintained above 50 mg/ml between infusions (Issell et al., 1978). If 1 g of mezlocillin is given as a ‘‘bolus’’ injection i.v. over 4–5 minutes to normal adults, serum levels are 56.2, 17.2, 2.9, and 0.1 mg/ml at 5 minutes, 30 minutes, 2 hours, and 6 hours, respectively.
Azlocillin
After a rapid i.v. injection of 1 g azlocillin (15 mg/kg body weight), the peak serum level at 5 minutes is 93 mg/ml, and the drug is undetectable in the serum at 8 hours. At the end of a 30-minute infusion of 5 g azlocillin (80 mg/kg), the serum level is 409 mg/ml, and it is still 2.6 mg/ ml 8 hours after the infusion.
c. Clinically important pharmacokinetic and pharmacodynamic features
Similar to other beta-lactams, the clinical efficacy of these agents is likely to be related to the duration the serum concentrations are above the MIC of the infecting pathogen.
In critically ill patients, a 2 g i.v. loading dose of piperacillin followed by continuous i.v. infusion of 8 g daily achieved percentage time above MIC of 100% and 65% for MICs of 16 and 32 mg/ml, respectively (Rafati et al., 2006). This was superior to intermittent infusion of 3 g i.v. every 6 hours, which achieved %TWMIC of 62% and 39%, respectively. The continuous-infusion regimen was associated with a more rapid decline in APACHE II scores in the first 4 days.
d. Excretion
Mezlocillin, azlocillin, and piperacillin are excreted unchanged in the urine by both glomerular filtration and tubular secretion. Approximately 50–80% of an i.v. dose of these drugs is eliminated via the kidneys in an unchanged form (Bergan, 1978; Fiegel and Becker, 1978; Meyers et al., 1980). With apalcillin only 18–20% of an administered dose is eliminated renally in the active form (Lode et al., 1984).
e. Drug interactions
With the exception of probenicid, which delays renal excretion of these penicillins, few interactions have been described. However, concurrent administration of piperacillin and methotrexate results in significantly decreased body clearance of methotrexate and its 7-OH metabolite (Najjar et al., 1998). Piperacillin at a dose of 1.5 and 3 g reduces the clearance of flucloxacillin by a factor of 1.5 and 2.1, respectively (Landersdorfer et al., 2008). Flucloxacillin has minimal effect on piperacillin pharmacokinetics. Similarly, in an animal model, renal clearance of imipenem was inhibited by piperacillin with minimal effect of imipenem on piperacillin excretion (Saitoh et al., 2006).
TOXICITY
a. Hypersensitivity reactions
Mezlocillin, azlocillin, and piperacillin may provoke any of the reactions which occur with penicillin G. These drugs are contraindicated in patients with a history of penicillin hypersensitivity. Parry and Neu (1982) evaluated 1148 mezlocillin-treated patients for adverse reactions. Hypersensitivity, manifested by drug fever, skin rashes, or eosinophilia, occurred in 0.3%, 1.8%, and 2.2% of treated patients, respectively. Eykyn (1982) reported one patient treated with azlocillin and tobramycin who had a severe hypersensitivity reaction after 18 days of therapy; she developed fever, malaise, rash, eosinophilia, and leukopenia, and recovered rapidly when both antibiotics were ceased.
b. Neurotoxicity
High doses of these drugs given i.v., similar to ‘‘massive’’ doses of penicillin G or carbenicillin, may have the propensity to cause neurotoxicity (Malanga et al, 1997).
c. Bleeding disorders
Similar to carbenicillin and ticarcillin, mezlocillin, azlocillin, piperacillin, and apalcillin can cause a disturbance of platelet function (Dijkmans et al., 1980; Gentry et al., 1981). Mezlocillin, piperacillin, and apalcillin have a lesser effect on platelet function than carbenicillin and ticarcillin at an equivalent dosage (Gentry et al., 1981; Copelan et al., 1983; Ballard et al., 1984; Gentry et al., 1985). d. Neutropenia and thrombocytopenia As with carbenicillin and ticarcillin and other beta-lactam antibiotics, reversible neutropenia can occur during therapy with mezlocillin, azlocillin, and piperacillin (Eykyn, 1982; Gooding et al., 1982; Parry and Neu, 1982).
e. Hepatotoxicity
Reversible hepatotoxicity, mainly manifested by elevated enzymes, such as serum alkaline phosphatase, SGOT, and SGPT, has been noted in 0.9% of mezlocillin-treated patients (Parry and Neu, 1982). One patient with severe cholestatic jaundice caused by mezlocillin has been reported (Hargreaves and Herchline, 1992).
f. Electrolyte and acid–base disturbance
An advantage of these penicillins is that their sodium content per gram is less than half that of carbenicillin and ticarcillin, thus decreasing the risk of fluid overload and hypokalemia when high doses are used (Eliopoulos and Moellering, 1982).
g. Other side-effects
Some patients have developed nausea and diarrhea associated with parenteral use of these drugs. Azlocillin and probably also other drugs of this group induce marked changes in colon microflora (Nord et al., 1986). A positive Coombs’ test has developed in a few patients treated by either mezlocillin or piperacillin, but hemolytic anemia has not been observed.
CLINICAL USES OF THE DRUG
Mezlocillin
This drug has no special advantages for the treatment of P. aeruginosa infections, but it has been used with success for the treatment of moderate and severe infections caused by other sensitive Gramnegative aerobic and anaerobic bacilli (Ellis et al., 1979; Thadepalli and Rao, 1979). Mezlocillin is quite effective in the treatment of septicemia, pneumonia, peritonitis, and infections of the urinary tract, skin and soft tissue, bone and joint, and the biliary tract caused by susceptible Gram-negative and Gram-positive aerobic and anaerobic bacteria (Pancoast et al., 1979; Konopka et al., 1982; Sanders, 1982b; Neu, 1982; Bjorvatn et al., 1983).
Azlocillin
Being more active than ticarcillin and mezlocillin in vitro against P. aeruginosa, this drug was mainly used for Pseudomonas infections. It was quite effective in serious P. aeruginosa infections, such as septicemia, meningitis, bronchopneumonia, and urinary tract infections (Ellis and Walter, 1979; Eykyn, 1982; Vestin et al., 1982; Neu et al., 1983). Azlocillin plus an aminoglycoside, such as tobramycin, is synergistic in vitro against P. aeruginosa, and in vivo it is satisfactory treatment for infections in neutropenic cancer patients (Klastersky et al., 1986; Gibson et al., 1989; Kibbler et al., 1989). A double betalactam combination of azlocillin plus ceftazidime was also satisfactory for the treatment of these patients (Kibbler et al., 1989).
b. Empiric treatment of febrile neutropenia
Piperacillin has been studied in combination with beta-lactamase inhibitors, aminoglycosides, ciprofloxacin, and cephalosporins for empiric treatment of febrile neutropenia. A detailed discussion of treatment of fever and neutropenia in combination with beta-lactamase inhibitor can be found in Chapter 17, Piperacillin–Tazobactam. In one small trial (50 infections) comparing piperacillin as monotherapy with carboxypenicillin–aminoglycoside as empiric treatment of serious bacterial infections, 30 patients with fever and neutropenia were included (Gribble et al., 1983). This study demonstrated that emergence of resistant organisms was more common during piperacillin therapy, which resulted in treatment failures and superinfections.
c. Bacteremia, lower respiratory tract infections, and other Gram-negative infections
Piperacillin is useful for the treatment of P. aeruginosa infections (Tosolini et al., 1985), but despite its superior in vitro activity, it has not been shown to be clinically superior to carbenicillin, ticarcillin, or ticarcillin–aminoglycoside combinations (Bodey et al., 1983; Grassi, 1985). Combination therapy with an aminoglycoside does not appear to prevent P. aeruginosa developing resistance (Nichols and Maki, 1985). Treatment of P. aeruginosa endocarditis with piperacillin– tobramycin has been associated with failure and in vivo development of piperacillin reistance (Watanakunakorn, 1984). Listeria monocytogenes bacteremia has also been successfully treated with piperacillin– amikacin (Hung et al., 1995).
d. Meningitis
Piperacillin monotherapy has been used successfully to treat meningitis in neonates and adults due to S. pneumoniae, Chryseobacterium (Flavobacterium) meningosepticum, and Achromobacter (Alcaligenes) xylosoxidans (Dickinson et al., 1981; Boo et al., 1989; Lin and Huang, 1991; Bruel et al., 1997; Kimura et al., 2005). As monotherapy and in combination with colistin, piperacillin has been successful in the treatment of meningitis due to P. aeruginosa, although failures have been reported with piperacillin montherapy (Dickinson et al., 1981; Karpuch et al., 1985).
References
Adler JL, Burke JP,Wilcox C, Finland M (1971). Susceptibility of Proteus species
and Pseudomonas aeruginosa to penicillins and cephalosporins. Antimicrob
Agents Chemother 1970: 63.
Alcaide F, Linares J, Pallares R et al. (1995). In vitro activities of 22 beta-lactam
antibiotics against penicillin-resistant and penicillin-susceptible viridans
group streptococci isolated from blood. Antimicrob Agents Chemother
39: 2243.
Aldridge KE, Ashcraft D, Cambre K et al. (2001). Multicenter survey of the
changing in vitro antimicrobial susceptibilities of clinical isolates of
Bacteroides fragilis group, Prevotella, Fusobacterium, Porphyromonas, and
Peptostreptococcus species. Antimicrob Agents Chemother 45: 1238.
Allan JD, Eliopoulos GM, Ferraro MJ, Moellering Jr RC (1985). Comparative
in vitro activities of cefpiramide and apalcillin individually and in
combination. Antimicrob Agents Chemother 27: 782.
Anaissie EJ, Fainstein V, Bodey GP et al. (1988). Randomized trial of betalactam
regimens in febrile neutropenic cancer patients. Am J Med 84: 581.
Anon (1994). Results of the North American trial of piperacillin/tazobactam
compared with clindamycin and gentamicin in the treatment of severe intraabdominal
infections. Investigators of the Piperacillin/Tazobactam Intraabdominal
Infection Study Group. Eur J Surg 573 (Suppl): 61.
Aoki M, Fukao T, Kaneko H et al. (2007). Clinical and bacteriological
evaluation of the efficacy of piperacillin in children with pneumonia. J Infect
Chemother 13: 224.
Appelbaum PC, Spangler SK, Jacobs MR (1991). Susceptibilities of 394
Bacteroides fragilis, non-B. fragilis group Bacteroides species, and Fusobacterium
species to newer antimicrobial agents. Antimicrob Agents Chemother
35: 1214.
Barry AL, Jones RN, Ayers LWet al. (1984). In vitro activity of apalcillin
compared with those of piperacillin and carbenicillin against 6797 bacterial
isolates from four separate medical centers. Antimicrob Agents Chemother
25: 669.
Barry AL, Jones RN, Thornsberry C (1985). Apalcillin (PC-904): spectrum of
activity and beta-lactamase hydrolysis/inhibition. Diagn Microbiol Infect Dis
3: 7.
Bassi O, Zuccarelli S, Amalfitano ME (1998). Clinical and economic outcomes
of empiric parenteral antibiotic therapy for pneumonia: a retrospective study
of 1032 hospitalized patients. J Chemother 10: 369.
Bell JM, Chitsaz M, Turnidge JD et al. (2007). Prevalence and significance of a
negative extended-spectrum beta-lactamase (ESBL) confirmation test result
after a positive ESBL screening test result for isolates of Escherichia coli and
Klebsiella pneumoniae: results from the SENTRY Asia-Pacific Surveillance
Program. J Clin Microbiol 45: 1478.
Bell SM, Pham JN, Lanzarone JYM (1985). Mutation of Pseudomonas aeruginosa
to piperacillin resistance mediated by beta-lactamase production.
J Antimicrob Chemother 15: 665.
Bergan T (1978). Pharmacokinetics of mezlocillin in healthy volunteers.
Antimicrob Agents Chemother 14: 801.
Bergan T (1981). Overview of acylureidopenicillin pharmacokinetics. Scand
J Infect Dis 29 (Suppl): 33.
Lastest Price from Mezlocillin manufacturers

US $0.00/kg2025-04-25
- CAS:
- 51481-65-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 51481-65-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt