Methyltrimethoxysilane: A Comprehensive Overview
Introduction
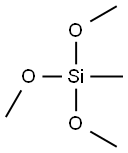
Methyltrimethoxysilane is a highly versatile organosilane that has garnered significant attention in various chemical industries due to its wide range of applications. As an organosilicon compound, methyltrimethoxysilane is particularly valuable for its ability to modify surfaces, act as a crosslinking agent, and contribute to the formation of durable films. Its molecular formula is CH₃Si(OCH₃)₃, and it is characterized by the presence of a silicon atom bonded to a methyl group and three methoxy groups. This unique structure enables METHYLTRIMETHOXYSILANE to participate in a variety of chemical reactions, making it a critical component in fields such as coatings, adhesives, sealants, and many more.

Figure 1 Characteristics of Methyltrimethoxysilane
Chemical Properties
Methyltrimethoxysilane is a clear, colorless liquid with a characteristic odor. Its molecular weight is 136.22 g/mol, and it has a boiling point of approximately 102°C (216°F). The compound is known for its high volatility and is soluble in most organic solvents such as alcohols, ketones, and hydrocarbons. However, it is not soluble in water under standard conditions. METHYLTRIMETHOXYSILANE has a flash point of around 10°C, indicating that it is highly flammable and requires careful handling during storage and transportation.
One of the key chemical properties of METHYLTRIMETHOXYSILANE is its ability to undergo hydrolysis in the presence of moisture, leading to the formation of silanols. These silanols can further condense to form siloxane bonds, which are essential for creating stable networks in materials such as resins and coatings. The hydrolysis reaction is exothermic, releasing methanol as a byproduct. This reactivity with water is a defining feature of METHYLTRIMETHOXYSILANE and underpins many of its industrial applications.
Composition and Structure
The chemical structure of Methyltrimethoxysilane consists of a silicon atom centrally bonded to a methyl group (-CH₃) and three methoxy groups (-OCH₃). This combination of organic and inorganic elements gives METHYLTRIMETHOXYSILANE its dual functionality: the methyl group provides hydrophobicity, while the methoxy groups are reactive sites for hydrolysis and condensation.
Upon hydrolysis, the methoxy groups are replaced by hydroxyl groups (-OH), transforming METHYLTRIMETHOXYSILANE into a silanol intermediate (CH₃Si(OH)₃). This intermediate can then undergo further condensation to form siloxane (Si-O-Si) bonds. The resulting siloxane network imparts mechanical strength, chemical resistance, and thermal stability to the final material, making METHYLTRIMETHOXYSILANE a crucial component in the formulation of various advanced materials.
Applications of Methyltrimethoxysilane
Surface Modification and Coatings
One of the primary applications of Methyltrimethoxysilane is in surface modification. METHYLTRIMETHOXYSILANE is commonly used as a coupling agent to enhance the adhesion between organic polymers and inorganic surfaces. This makes it ideal for use in coatings, where it helps to improve the durability and weather resistance of the surface. The ability of METHYLTRIMETHOXYSILANE to form a hydrophobic layer also makes it valuable in the creation of water-repellent coatings, which are used in the construction, automotive, and electronics industries.
Sealants and Adhesives
METHYLTRIMETHOXYSILANE plays a critical role in the formulation of sealants and adhesives, particularly in systems where moisture resistance and long-term durability are required. The compound’s ability to crosslink and form strong siloxane bonds contributes to the development of adhesives that can withstand harsh environmental conditions. This makes METHYLTRIMETHOXYSILANE an essential component in the production of construction sealants, automotive adhesives, and industrial bonding agents.
Sol-Gel Processing
In sol-gel chemistry, METHYLTRIMETHOXYSILANE is used as a precursor for the production of silica-based materials. The hydrolysis and condensation reactions of METHYLTRIMETHOXYSILANE lead to the formation of silica gels, which can be processed into coatings, films, and other advanced materials. Sol-gel processing is widely employed in the production of optical coatings, catalysts, and ceramics. The versatility of METHYLTRIMETHOXYSILANE in sol-gel applications has made it a popular choice for researchers and manufacturers looking to create materials with tailored properties.
Silicone Resins
Methyltrimethoxysilane is a key ingredient in the production of silicone resins, which are used in a variety of high-performance applications. Silicone resins are known for their excellent thermal stability, electrical insulation properties, and resistance to weathering. These characteristics make them suitable for use in coatings, varnishes, and electrical insulation materials. METHYLTRIMETHOXYSILANE contributes to the crosslinking of the resin network, enhancing its mechanical properties and overall performance.
Environmental Applications
METHYLTRIMETHOXYSILANE is also used in environmental applications, particularly in the treatment of surfaces to make them water-resistant or to modify their chemical properties. For instance, METHYLTRIMETHOXYSILANE can be applied to building materials to prevent water absorption and reduce the degradation caused by moisture. Additionally, METHYLTRIMETHOXYSILANE is being explored for use in the treatment of textiles and paper products to impart hydrophobic properties, thereby extending their lifespan and improving their performance in challenging environments.
Storage and Handling of Methyltrimethoxysilane
Proper storage and handling of Methyltrimethoxysilane are crucial to maintaining its stability and preventing accidents. Due to its flammability and volatility, METHYLTRIMETHOXYSILANE should be stored in a cool, well-ventilated area, away from sources of ignition. The compound should be kept in tightly sealed containers made of materials that are compatible with organosilanes, such as stainless steel or certain plastics.
METHYLTRIMETHOXYSILANE is sensitive to moisture, and exposure to water can initiate hydrolysis, leading to the formation of methanol and potentially hazardous conditions. Therefore, it is essential to store METHYLTRIMETHOXYSILANE in a dry environment and minimize its contact with water or humid air. When handling METHYLTRIMETHOXYSILANE, proper personal protective equipment (PPE), including gloves, safety goggles, and flame-resistant clothing, should be worn to reduce the risk of exposure and accidents.
In industrial settings, METHYLTRIMETHOXYSILANE is typically handled in closed systems to prevent the release of vapors into the atmosphere. Ventilation systems should be in place to control the concentration of vapors and ensure a safe working environment. Additionally, appropriate fire suppression systems should be readily available in areas where METHYLTRIMETHOXYSILANE is stored or used, given its flammability.
[1] Rao A V, Kulkarni M M, Amalnerkar D P, et al. Superhydrophobic silica aerogels based on methyltrimethoxysilane precursor[J]. Journal of Non-Crystalline Solids, 2003, 330(1-3): 187-195.
[2] Nadargi D Y, Rao A V. Methyltriethoxysilane: New precursor for synthesizing silica aerogels[J]. Journal of alloys and compounds, 2009, 467(1-2): 397-404.
References:
[1] A. VENKATESWARA RAO . Superhydrophobic silica aerogels based on methyltrimethoxysilane precursor[J]. Journal of Non-crystalline Solids, 2003, 330 1: 1-286. DOI:10.1016/j.jnoncrysol.2003.08.048.[2] DIGAMBAR Y. NADARGI A V R. Methyltriethoxysilane: New precursor for synthesizing silica aerogels[J]. Journal of Alloys and Compounds, 2009, 467 1: L1-L26. DOI:10.1016/j.jallcom.2007.12.019.
You may like
Related articles And Qustion
See also
Lastest Price from Methyltrimethoxysilane manufacturers

US $0.00-0.00/KG2025-12-22
- CAS:
- 1185-55-3
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00/kg2025-06-13
- CAS:
- 1185-55-3
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 500mt per month