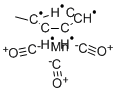Methylcyclopentadienyl Manganese Tricarbonyl: Plant Uptake, Effects on Metabolism and Neurotoxic Effects
General Description
Methylcyclopentadienyl manganese tricarbonyl, an organic compound used in gasoline, leads to manganese oxide deposition along North American roadsides, increasing manganese uptake by plants, especially in low-oxygen environments. Plant responses to Methylcyclopentadienyl manganese tricarbonyl vary, with differing toxicity symptoms and metabolic effects across species. Research shows that Methylcyclopentadienyl manganese tricarbonyl influences plant metabolism, impacting respiration, substrate utilization, and growth, particularly in radishes. Moreover, Methylcyclopentadienyl manganese tricarbonyl induces neurotoxic effects, causing seizure activity in mice by inhibiting the GABA-A receptor linked chloride channel. Understanding these complex plant interactions with Methylcyclopentadienyl manganese tricarbonyl and manganese requires further research for comprehensive implications on plant health and ecosystem dynamics.

Figure 1. Methylcyclopentadienyl manganese tricarbonyl
Plant uptake
Methylcyclopentadienyl manganese tricarbonyl (C9H7MnO3) is an organic compound containing manganese, utilized in gasoline to enhance octane ratings and act as an antiknock agent. When Methylcyclopentadienyl manganese tricarbonylcontaining gasoline is combusted, it produces manganese oxides, which are subsequently deposited along roadsides in North America in correlation with traffic density. As a result, plants in these areas can absorb the manganous ion, leading to elevated concentrations of manganese. This uptake is particularly prominent in scenarios where the root and soil environment lacks oxygen, such as in submerged or emergent plants. It's worth noting that at moderate concentrations, visible toxicity symptoms may vary between plant species due to various factors. For instance, lead and cadmium can inhibit transpiration and photosynthesis while simultaneously stimulating the synthesis of respiration and abscisic acid. These varying responses to Methylcyclopentadienyl manganese tricarbonyl uptake highlight the complexity of plant interactions with manganese and other metals, demonstrating the need for further research to fully understand the implications of Methylcyclopentadienyl manganese tricarbonyl exposure on plant health and ecosystem dynamics. 1
Effects on metabolism
The effects of Methylcyclopentadienyl manganese tricarbonyl on plant metabolism are complex and multifaceted. Tissue manganese concentration, influenced by both the metal's concentration and exposure time, interacts with temperature to impact respiration and overall metabolic processes. Research spanning 70 years has revealed that air pollutants, including Methylcyclopentadienyl manganese tricarbony, can lead to prelethal and reversible damage in plants, manifesting as an increase in metabolism known as wound respiration. This heightened metabolic activity likely reflects the synthesis of defense mechanisms within the plant. Notably, Methylcyclopentadienyl manganese tricarbony and other pollutants do not selectively target a specific enzyme or metabolite; instead, they initiate damage by causing the peroxidation of fatty acids in membranes, ultimately leading to cell death. The impact of Methylcyclopentadienyl manganese tricarbony on radish metabolism, respiration, substrate utilization efficiency, and growth is temperature-dependent and, to some extent, time-dependent. Additionally, a decrease in dry-matter production has been observed at varying leaf manganese levels, highlighting the intricate relationship between manganese concentration and plant activity. These findings suggest that studying plant stress induced by manganese using microcalorimetry could yield valuable insights into the metabolic effects of Methylcyclopentadienyl manganese tricarbonyl exposure. 1
Neurotoxic effects
Methylcyclopentadienyl manganese tricarbonyl has been found to induce neurotoxic effects, particularly seizure activity, in mice. When administered in propylene glycol or corn oil, Methylcyclopentadienyl manganese tricarbonyl triggered seizures in the mice, with a lower LD50 associated with seizure activity observed in mice receiving Methylcyclopentadienyl manganese tricarbonyl in propylene glycol compared to those receiving it in corn oil. Furthermore, the concentration of manganese in the brains of mice experiencing seizure activity due to Methylcyclopentadienyl manganese tricarbonyl was notably higher than in those that did not exhibit seizures. Interestingly, mice treated with manganese chloride (MnCl2) showed similar increases in brain manganese levels but did not display seizure activity. Additionally, Methylcyclopentadienyl manganese tricarbonyl at non-lethal seizure-inducing doses did not affect the accumulation of 4-aminobutyric acid (GABA) in the mouse brain. However, it did inhibit the binding of t-[3H] t-butylbicycloorthobenzoate [3H]-TBOB, a ligand for the GABA-A-receptor linked chloride channel, in mouse brain membranes. This suggests that MMT or a related metabolite, rather than elemental manganese itself, is likely responsible for the observed seizure activity. The data also indicate that the seizure activity may result from Methylcyclopentadienyl manganese tricarbonyl's inhibitory effect on the GABA-A receptor linked chloride channel. 2
Reference
1. Jones AR, Lytle CM, Stone RL, et al. Methylcyclopentadienyl manganese tricarbonyl (MMT), plant uptake and effects on metabolism. Thermochimica Acta, 2000, 349(1):141-146.
2. Fishman BE. Neurotoxic effects of methylcyclopentadienyl manganese tricarbonyl (MMT) in the mouse: basis of MMT-induced seizure activity. Toxicology, 1987, 45(2):193-201.
Related articles And Qustion
Lastest Price from Methylcyclopentadienyl manganese tricarbonyl manufacturers

US $1.00/KG2025-04-21
- CAS:
- 12108-13-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10mt

US $6.00/kg2025-04-21
- CAS:
- 12108-13-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month