Methylamine Hydrochloride: A Comprehensive Overview for Chemical Professionals
Introduction
Methylamine Hydrochloride is a derivate of methylated amine which could be used as nitrogen source to induced the FLD expression.Vukic´evic et al have established a convenient onepot solvent-free synthesis of N-methyl imines from aromatic aldehydes, using methylamine hydrochloride as the methylamine source.

Structure
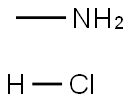
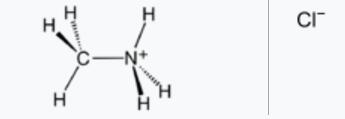
Methylamine hydrochloride CH5N·HCl crystallizes in the tetragonal system, space group P4/nmm (129) with lattice parameters a=b= 0.6068(1) nm and c= 0.50689(8) nm[1].
Synthesis
Each may be prepared as its hydrochloride by methylating ammonium chloride with formaldehyde under suitable conditions. Methylamine hydrochloride is easy and safe to handle. It could be either commercially obtained or prepared by a very simple procedure from an inexpensive formaldehyde solution and ammonium chloride[2].
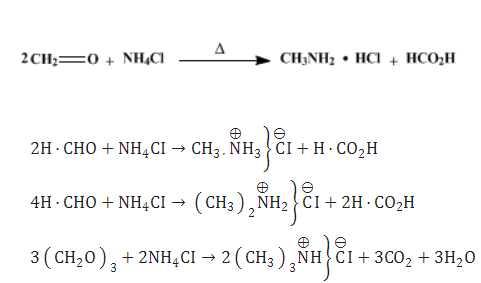
Methylamine hydrochloride is obtained by heating two equivalents of formaldehyde (as formalin) with ammonium chloride at about 100°. Any dimethylamine hydrochloride formed may be removed by extraction with chloroform.
Dimethylamine hydrochloride is the main product when four equivalents of formaldehyde and a somewhat higher temperature are used.
With a large excess of formaldehyde (as paraformaldehyde) at 160°, trimethylamine hydrochloride is obtained in good yield.
![Article illustration]() References
References
[1] B. Lasocha, W. Lasocha, B. Gaweł. “Powder diffraction investigations of some organic hydrochlorides.” Powder Diffraction 21 1 (2006): 310–313.
[2] Niko S. Radulović , Rastko D. Vukićević, Ana B. Miltojević . “Simple and efficient one-pot solvent-free synthesis of N-methyl imines of aromatic aldehydes.” 2013. Pages 257-270.
Related articles And Qustion
See also
Lastest Price from Methylamine hydrochloride manufacturers

US $10.00-1.00/kg2025-11-03
- CAS:
- 593-51-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 300tons

US $10.00/ASSAYS2025-05-04
- CAS:
- 593-51-1
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 tons