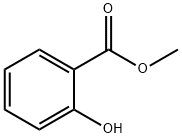
Methyl Salicylate: Synthesis, Composition, Applications, and Storage Insights
Introduction
Methyl salicylate, a chemical compound that might seem obscure to the layperson, is a pervasive ingredient in various everyday products, from medicines to fragrances. Known chemically as C₈H₈O₃, this ester of salicylic acid is not just a simple compound but a significant player in the pharmaceutical and cosmetic industries. Its distinctive minty aroma and pain-relieving properties make it a favorite in formulations designed to soothe muscles and joints. Furthermore, its role extends into agricultural products, serving as a key component in various eco-friendly pesticides. This versatility not only makes it invaluable but also highlights its complex interaction with biological and ecological systems[1].

Figure 1 Characteristics of Methyl salicylate
Synthesis of Methyl Salicylate
The synthesis of methyl salicylate predominantly involves the esterification of salicylic acid with methanol. This reaction is typically catalyzed by an acid such as sulfuric acid or HCl, which serves to accelerate the reaction by donating a proton to the oxygen of the carboxylic group, thereby facilitating the departure of the hydroxyl group as water and forming the ester bond. This synthesis method is favored for its straightforwardness and cost-effectiveness, making it suitable for large-scale industrial production.
Main Components
Methyl salicylate is a benzoate ester that is structurally composed of a benzene ring with adjacent hydroxyl (OH) and methoxy (OCH₃) groups. The compound's molecular structure contributes to its distinct properties such as its anti-inflammatory, analgesic, and aromatic characteristics. Its purity and chemical consistency are critical, especially when used in pharmaceutical applications, necessitating stringent quality control during production.
Uses of Methyl Salicylate
Methyl salicylate's applications are vast and varied. Medically, it is incorporated in topical pain relievers, where it acts as a counterirritant to distract the brain from deeper-seated pains via superficial cooling or warming sensations. In dentistry, it is used in root canal procedures as a disinfectant. Its analgesic properties are crucial for products aimed at alleviating minor aches and arthritis symptoms, making it a key ingredient in over-the-counter muscle rubs and patches.
Beyond healthcare, methyl salicylate is a staple in the flavoring and fragrance industry. It imparts a pleasant wintergreen scent to various products, ranging from mouthwashes and kinds of toothpaste to chewing gums and breath fresheners. Furthermore, its inclusion in plant-based insecticidal products showcases its versatility, serving as a testament to its efficacy in non-medical realms. In the cosmetic industry, it enhances the sensory appeal of lotions and perfumes. Its robust profile also makes it effective in masking odors in household cleaning products and industrial applications where odor control is necessary. The broad range of its applications reflects its integral role in not only health and wellness but also in enhancing everyday consumer experiences.
Storage Recommendations
The storage of methyl salicylate must be handled with care to preserve its efficacy and safety. It should be kept in a cool, well-ventilated area away from direct sunlight and heat sources, as it is susceptible to degradation when exposed to extreme temperatures. Containers of methyl salicylate need to be tightly sealed to prevent leakage and contamination. Since it is slightly volatile and has a strong odor, proper sealing also helps control its pervasive scent. Moreover, safety guidelines recommend storing it away from oxidizing agents and bases, as adverse reactions can occur under such conditions.
Conclusion
Methyl salicylate is more than just an ingredient; it is a multifaceted compound with broad applications that underscore its significance in the chemical, pharmaceutical, and consumer products sectors. Understanding its synthesis, composition, and proper storage methods not only facilitates its effective use but also underscores the importance of chemical safety protocols. As research progresses, the potential for new applications of methyl salicylate continues to expand, promising further advancements in both its industrial and therapeutic uses. This continued evolution cements its place as a crucial substance in the scientific community, making its study and application an exciting frontier for chemistry professionals[2].
References
[1]Park S W, Kaimoyo E, Kumar D, et al. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance[J]. Science, 2007, 318(5847): 113-116.
[2]Shulaev V, Silverman P, Raskin I. Airborne signalling by methyl salicylate in plant pathogen resistance[J]. Nature, 1997, 385(6618): 718-721.
Related articles And Qustion
See also
Lastest Price from Methyl salicylate manufacturers

US $1.00-4.00/KG2025-09-12
- CAS:
- 119-36-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG

US $2.00/kg2025-06-10
- CAS:
- 119-36-8
- Min. Order:
- 10kg
- Purity:
- 99%
- Supply Ability:
- 10000kg