Methyl glycolate: Overview, Conversion of Glucose to Methyl Glycolate and Safety Considerations
General Description
Methyl glycolate is a versatile and important compound used extensively in the chemical industry as an intermediate for esters, plastics, and solvents. It also has potential applications in biofuels and biodegradable polymers. The conversion of glucose to methyl glycolate via methanolysis is a promising green method for its synthesis. Researchers have shown that adding acids with oxidizing properties, such as phenolic acids, can enhance the efficiency of the reaction. However, it is important to handle methyl glycolate with care as it is classified as a combustible liquid and can cause skin, eye, and respiratory irritation. Proper safety precautions, including using personal protective equipment and storing away from heat and flames, must be taken when working with this compound.

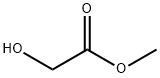
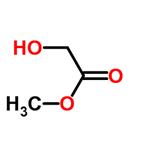
Figure 1. Methyl glycolate
Overview
Methyl glycolate is an organic compound that has the chemical formula C3H6O3. It is a colorless liquid with a slight odor and can easily mix with water and various organic solvents. This compound is primarily utilized as an intermediate in the chemical industry, where it plays a crucial role in the production of esters, plastics, and solvents. The applications of methyl glycolate extend beyond the chemical industry. It holds promise in the field of biofuels, as it can be converted into biodiesel and bioethanol, providing a renewable and sustainable energy source. Researchers are also exploring its potential in the synthesis of biodegradable polymers, which have significant environmental benefits compared to traditional plastics. Moreover, methyl glycolate serves as a valuable feedstock for the production of chemicals and fuels through biocatalytic processes. These processes leverage the power of enzymes or microorganisms to carry out chemical transformations, offering greener alternatives to conventional chemical synthesis methods. In summary, methyl glycolate is a versatile compound with diverse applications. Its role as an intermediate in the chemical industry, potential use in biofuels and biodegradable polymer synthesis, and its value as a feedstock for biocatalytic processes highlight its significance in various sectors. 1
Conversion of glucose to methyl glycolate
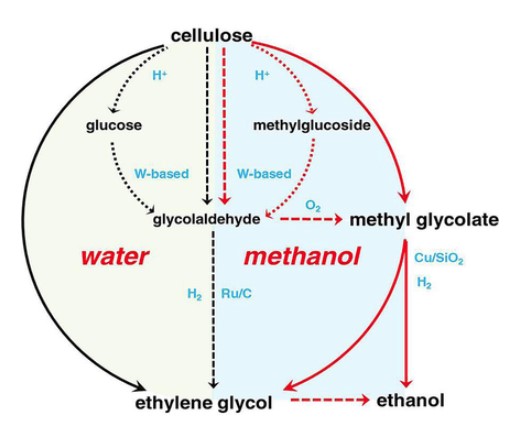
Methyl glycolate is a valuable biodegradable plastic monomer, and there is growing interest in developing green and sustainable approaches for its synthesis. One promising method involves converting glucose to Methyl glycolate via methanolysis, a process that involves breaking down the glucose molecule with methanol. Researchers have developed a green approach to this process by combining subcritical methanol with a phenol/quinone redox system. This combination promotes the reversible cleavage of C-C bonds and oxidation during the cascade reaction of glucose to Methyl glycolate. The researchers investigated the methanolysis of glucose in detail and found that the reaction yielded 38% Methyl glycolate at a temperature of 240°C under a methanol solution in an air atmosphere. Interestingly, they also discovered that adding a trace amount of water facilitated the production of the targeted product. To enhance the efficiency of the reaction, the researchers added acids with oxidizing properties to promote the oxidation of the thermally unstable intermediate and enhance the C-C cleavage. Phenolic acids were found to perform particularly well, with tyrosine yielding 64.0% Methyl glycolate. In summary, the selective methanolysis of glucose is a promising green method for the synthesis of Methyl glycolate. The use of subcritical methanol and a phenol/quinone redox system, along with the addition of phenolic acids, enhances the efficiency of the reaction and offers a sustainable route for the production of this valuable compound. 2
Safety
Methyl glycolate is classified as a combustible liquid, posing risks of skin and eye irritation, as well as potential respiratory irritation. Precautionary measures include handling in well-ventilated areas, using appropriate personal protective equipment, and avoiding contact with skin, eyes, and clothing. The substance should be stored away from heat, sparks, and open flames. It is important to note that the safety information may vary depending on impurities and additives present in the product. Overall, proper care and adherence to safety guidelines are crucial when working with methyl glycolate. 1
Reference
1. Methyl glycolate. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 66774.
2. Weng Y, Zou M, Liu X, et al. Conversion of glucose to methyl glycolate in subcritical methanol. Chem Commun (Camb). 2023;59(29):4340-4343.
Related articles And Qustion
See also
Lastest Price from Methyl glycolate manufacturers
US $0.00-0.00/kg2025-11-20
- CAS:
- 96-35-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons

US $30.00-10.00/KG2025-04-15
- CAS:
- 96-35-5
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg