The introduction of Flupyrimin
Description
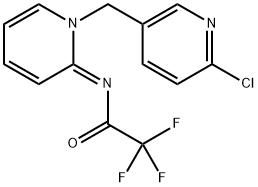
Flupyrimin (FLP) [N-[(E)-1-(6-chloro-3-pyridinylmethyl)pyridin-2(1H)-ylidene]-2,2,2-trifluoroacetamide] is a novel chemotype insecticide. It was announced by Meiji Seika Pharma as a new insecticide 2017. This new neonicotinoid insecticide has unique biological properties, including outstanding potency to imidacloprid (IMI)-resistant rice pests and superior safety toward pollinators.
Flupyrimin can effectively control the main pests of rice fields, is relatively safe for pollinating insects such as bees, and has huge market potential[1]. Flupyrimin is persistent, stable to the environment, and has a hydrolysis half-life of 228 days (25 °C, pH 7), which may pose a risk to surface water and ecosystems. Flupyrimin may enter the human body through the food chain directly or indirectly, potentially endangering human health. However, there is no information on the interaction between flupyrimin and nitenpyram and essential physiological proteins.
Biological activity
FLP acts as a nicotinic antagonist in American cockroach neurons, and [3H]FLP binds to the multiple high-affinity binding components in house fly nicotinic acetylcholine (ACh) receptor (nAChR) preparation. One of the [3H]FLP receptors is identical to the IMI receptor, and the alternative is the IMI-insensitive subtype. Furthermore, FLP is favorably safe to rats as predicted by the very low affinity to the rat α4β2 nAChR. It is, just like the structurally related imidacloprid, acting on the nicotinic acetylcholine receptor[2]. However, flupyrimin binds as an antagonist via a recognition manner different from the established neonicotinoid insecticides and, therefore, displays potent activity towards imidacloprid-resistant rice pests, such as Nilaparvata lugens (brown planthopper) and Sogatella furcifera (white-backed planthopper), while at the same time being safe towards pollinators.
![N-[(2E)-1-[(6-chloropyridin-3-yl)methyl]pyridin-2 (1H)-ylidene]-2,2,2-trifluoroacetamide Article illustration](/NewsImg/2024-02-23/6384428191120570973740962.png)
Synthesis method
The synthesis of flupyrimin starts with the acylation of 2-aminopyridine (146) with ethyl trifluoroacetate, which delivers, under strongly basic conditions, the sodium nitride derivative 147. This ionized amide is then alkylated with 2-chloro-5-(chloromethyl)pyridine, a well-known neonicotinoid building block, to deliver flupyrimin[3].
![N-[(2E)-1-[(6-chloropyridin-3-yl)methyl]pyridin-2 (1H)-ylidene]-2,2,2-trifluoroacetamide Article illustration](/NewsImg/2024-02-23/6384428201164425536924320.png)
References
[1] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.
[2] Zongyuan Zhao . “Comparison of the interactions of flupyrimin and nitenpyram with serum albumins via multiple analysis methods.” Chemosphere 289 (2022): Article 133139.
[3] Yasumichi Onozaki. “Flupyrimin: A Novel Insecticide Acting at the Nicotinic Acetylcholine Receptors.” Journal of Agricultural and Food Chemistry 65 36 (2017): 7865–7873.