Methyl benzenesulfonate: properties and applications
General Description
Methyl benzenesulfonate is a yellow liquid with a pungent odor. It has excellent water solubility and stability, making it suitable for storage and transportation. With a relatively high boiling point, it can withstand moderate temperatures without decomposing. It is commonly used in various industrial applications due to its water solubility, stability, moderate temperature resistance, and mild odor. Some of its applications include ethylene polymerization, methylation of 3-methylxanthine, and the preparation of thin cation-exchanger films. In ethylene polymerization, it is used to synthesize imine ligands and palladium complexes for the polymerization of ethylene. In the methylation of 3-methylxanthine, it acts as a methylating agent to transfer a methyl group and synthesize theobromine. In the preparation of thin cation-exchanger films, it is used to create films with high permselectivity and low resistance for efficient ion transportation and conductivity.

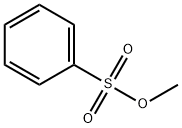
Figure 1. Methyl benzenesulfonate
Properties
Methyl benzenesulfonate is a yellow liquids with a pungent odor with the chemical formula of C7H8O3S. It exhibits excellent water solubility due to its strong sulfonic acid group. The compound demonstrates good stability, making it suitable for storage and transportation. With a relatively high boiling point, it can withstand moderate temperatures without decomposing. Additionally, it has a mild odor, making it easier to handle and reducing unpleasant smells in its applications. These properties, including water solubility, stability, moderate temperature resistance, and mild odor, contribute to the versatility of methyl benzenesulfonate in various industrial applications. 1
Applications
Ethylene Polymerization
Methyl benzenesulfonate has been utilized in the polymerization of ethylene, as demonstrated by the synthesis of various imine ligands and their corresponding palladium complexes. The reaction between sodium 2-formylbenzenesulfonate and aniline resulted in the formation of sodium-2-((phenylimino)methyl)benzenesulfonate L1 with a near-quantitative yield of 91%. Other imine ligands were generated by treating various electron-donating amines with sodium 2-formylbenzenesulfonate, resulting in a library of 6 imine ligands (L2–L6). Palladium complexes such as [L2PdMe(Lu)] C1, [L4/5PdMe(Lu)]-type complexes C2–C3 were also synthesized and successfully utilized in the insertion polymerization of ethylene to polyethylene. The formation of these complexes was ascertained using a combination of spectroscopic tools, analytical methods, and single-crystal X-ray diffraction. The successful application of these complexes in the polymerization of ethylene demonstrates their synthetic utility in the field of materials science. 2
Methylation of 3-methylxanthine
In the process of preparing theobromine from 3-methylxanthine, methyl benzenesulfonate plays a crucial role as the methylating agent. The methylation reaction involves the transfer of a methyl group from methyl benzenesulfonate to the 3-methylxanthine molecule, leading to the formation of the desired theobromine compound. Methyl benzenesulfonate is chosen as the methylating agent due to its ability to effectively transfer the methyl group under suitable reaction conditions. It serves as a source of the methyl group that is necessary for the methylation process. By carefully controlling the reaction conditions such as temperature, solvent, and reaction time, the methylation of 3-methylxanthine with methyl benzenesulfonate can selectively occur, resulting in the synthesis of theobromine. Theobromine is an important compound used in various applications, including the production of pharmaceuticals, food and beverages, and cosmetics. Overall, methyl benzenesulfonate acts as a methylating agent in the methylation of 3-methylxanthine, facilitating the synthesis of theobromine with its methyl group transfer capability. 3
Preparation of thin cation-exchanger films
Methyl benzenesulfonate finds application in the preparation of thin cation-exchanger films, which are characterized by their ultrathin, uniform, and pinhole-free nature. These films are formed through plasma polymerization of 1,3-butadiene along with methyl benzenesulfonate, resulting in the incorporation of sulfonic ester groups. To further enhance the functionality of these films, the sulfonic ester groups are transformed into lithium sulfonate by reacting the plasma polymer with lithium iodide. The resulting sulfonate cation-exchange films exhibit remarkable permselectivity, demonstrated by a high K+ ion transference number of 0.99 in aqueous KCl solutions. Additionally, they display low resistance per unit area, with a value of 0.04 ω-cm2 in 0.5M aqueous H2SO4, indicating favorable ionic conductivity of 1.8 × 10-4 S cm-1. Overall, the utilization of methyl benzenesulfonate in the formation of thin cation-exchanger films offers a promising approach to creating superior-quality films with excellent permselectivity and low resistance for applications that require efficient ion transportation and conductivity. 4
Reference
1. PubChem. Compound Summary: Methyl benzenesulfonate. National Library of Medicine, 2005, CID:6630.
2. Deshmukh SS, Gaikwad SR, Mote NR, M M, Gonnade RG, Chikkali SH. Neutral Imino-Methyl Benzenesulfonate-Ligated Pd(II) Complexes and Implications in Ethylene Polymerization. ACS Omega, 2019, 4(5):9502-9511.
3. Nesterov VM, Chubova IE. Methylation of 3-methylxanthine with methyl benzenesulfonate. Pharmaceutical Chemistry Journal, 1967, 1(1):38-38.
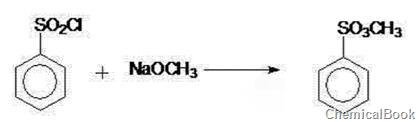
4. Yoshiharu U, Yoshiharu U, Kazuaki Y, Zempachi O, Zen-ichiro T. Thin cation-exchanger films by plasma polymerization of 1,3-butadiene and methyl benzenesulfonate. Journal of The Electrochemical Society, 1991, 138(11):3190-3193.
Related articles And Qustion
See also
Lastest Price from Methyl benzenesulfonate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 80-18-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $10.00/kg2025-04-21
- CAS:
- 80-18-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 ton
![60511-85-5 11,12-Dihydroindolo[2,3-a]carbazoleindolo[2,3-a]carbazoleIndocarbazoleapplication](/NewsImg/2024-12-17/6387003739472769161235461.jpg)
![60511-85-5 11,12-Dihydroindolo[2,3-a]carbazoleindolo[2,3-a]carbazoleIndocarbazoleapplication](/NewsImg/2023-09-11/6383004311714241575532394.jpg)
