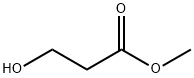
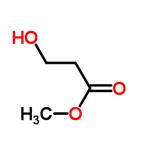
Methyl 3-Hydroxypropanoate: Catalytic Synthesis from Ethylene Oxide and Thermolysis Behavior
Methyl 3-hydroxypropanoate is an alkyl/ether-based PROTAC linker that can be used in the synthesis of PROTACs.It is an organic compound with the chemical formula C4H8O3 and the structural formula CH3OCH2C(O)CH2OH. Methyl 3-hydroxypropanoate can be prepared by formic acid esterification, that is, the reaction of 3-hydroxypropionic acid with methanol, usually under acidic conditions.

Efficient catalytic carbonylation of ethylene oxide into methyl 3-hydroxypropanoate
Since the ring-opening carbonylation of epoxide in alcohol solvent has high atomic economy, it is very attractive to prepare downstream high value-added products using epoxide as the reactant via catalytic technique. Among the most commonly used epoxides, ethylene oxide (EO) could be obtained as a petrochemical product in excess capacity by industrial production. It also becomes a promising raw material to produce Methyl 3-hydroxypropanoate (3-HPM) through carbonylative conversion, with the subsequent hydrogenation product 1,3-propanediol (1,3-PDO) as one of the most expected industrial products for new polyester (PTT) and medical intermediates. No matter how, the industrial path for perspective 3-HPM and 1,3-PDO required the key carbonylative transformation of EO with high efficiency and selectivity, which was, however, greatly challenging until current days. In this work, Co2(CO)8 was first used to catalyze the carbonylative transformation of EO to Methyl 3-hydroxypropanoate. It is necessary to mention that the Co/CNT-C sample was selective toward the transformation of EO to Methyl 3-hydroxypropanoate with a similar atomic efficiency to that of Co2(CO)8 cooperated with 3-hydroxypyridine. The key factors for the enhanced catalytic performances were interpreted by combining the initial properties of Co/CNT by using the conventional impregnation method.[1]
The carbonylative transformation of ethylene oxide (EO) was catalyzed by using the cobalt catalyst in the presence of CO. Methanol was used in excess as both solvent and reactant. Compared with Co2(CO)8 as catalyst, inorganic salts such as Co(OAc)2 and Co(NO3)2 displayed poor EO conversion of only 2.3% and 2.9%, respectively. Also, no Methyl 3-hydroxypropanoate (3-HPM) was detected in the reaction mixture. In this work, carbonylative transformation of EO was investigated using commercial Co2(CO)8 in a homogeneous system to produce 3-HPM. The reaction parameters including dosages of reactants, cooperated ligands, and CO pressure were optimized to understand the chemical kinetics of EO carbonylation. The specific rate based on per gram Co per hour via Co/CNT-C was 52.2 mmol.g−1Co.h−1 , although the macroscopical EO conversion still needs work in progress. The Co/CNT-C sample was highly selective toward Methyl 3-hydroxypropanoate with selectivity of 92.5% and a similar atomic efficiency to that of Co2(CO)8 cooperated with 3-hydroxypyridine. Most important of all, the immobilized Co particles on Co/CNT-C enabled the facile separation and recycling for potential applications of EO carbonylation.
The thermolysis of Methyl 3-hydroxypropanoate in m-xylene solution
Methyl 3-hydroxypropanoate belongs to the family of β-hydroxycompounds, which are structural units of significant interest in organic synthesis. This family also includes β-hydroxyketones, β-hydroxyolefins, β-hydroxyacetilenes and β-hydroxyesters. The thermolysis of many of these compounds has been previously studied. Furthermore, it is well known that optically active hydroxycarboxylates have application as chiral synthons and monomers for the synthesis of biodegradable polymers. This procedure combine quantum mechanical methods for the calculation of configuration energies with statistical thermodynamics for the elucidation of molecular association process. The reminder of this article is organized as follows: in Section 2 we describe our calculation set-up with the application of explicit and implicit models in the study the effect of m-xylene solvent in the thermolysis of Methyl 3-hydroxypropanoate, with emphasis in the rate-limiting step of the chemical reaction.[2]
The solvent effects on the thermolysis of Methyl 3-hydroxypropanoate have been determined through a combination of continuum and explicit models. The application of the SCIPCM model showed a value of the reaction rate constant in agreement with the experiment as well as with the value in the gas phase. The application of MMH procedure improved the understanding of the reaction with the explicit addition of m-xylene molecules. The most important association clusters for the solute–solvent interactions provide information about the stabilization of reactant, products and transition state structures by weakly interactions. The presence of m-xylene molecules does not imply any electronic effect on the transition state structure. The association thermodynamic quantities indicate that the reaction is favored in m-xylene solution. MMH procedure could be a useful method to contribute to the understanding of the solvent effects on chemical reactions.
References
[1]Luo J, Liu P, Yang W, Niu H, Li S, Liang C. Chemical kinetics and promoted Co-immobilization for efficient catalytic carbonylation of ethylene oxide into methyl 3-hydroxypropionate. Front Chem. 2022 Jul 22;10:945028. doi: 10.3389/fchem.2022.945028. PMID: 35936085; PMCID: PMC9354985.
[2]Rodríguez‐Linares, D., Codorniu‐Hernández, E., Velez‐Ortíz, E., Murillo‐López, J. A., Villegas‐Bolaños, P. A., & Quijano‐Tobón, J. (2009). Theoretical study on the thermolysis of methyl‐3‐hydroxypropanoate in m‐xylene solution. Theochem, 902(1-3), 41-48.
See also
Lastest Price from Methyl 3-hydroxypropanoate manufacturers
US $8.80-2.20/kg2025-06-28
- CAS:
- 6149-41-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg

US $1.00-1.00/kg2025-03-31
- CAS:
- 6149-41-3
- Min. Order:
- 1kg
- Purity:
- 0.98+
- Supply Ability:
- 20 tons