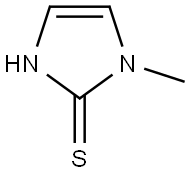
Methimazole: an antithyroid medication
Methimazole is an antithyroid medication which is now considered the first line agent for medical therapy of hyperthyroidism and Graves disease. Methimazole has been linked to serum aminotransferase elevations during therapy as well as to a clinically apparent, idiosyncratic liver injury that is typically cholestatic and self-limited in course.

Synthesis of Methimazole
N-methylimidazole 8.21 g (100 mmol) and 80 ml of tetrahydrofuran were added to a three-necked reaction flask and then cooled to -5 ° C. A solution of n-butyllithium-n-hexane (50 ml, n-butyllithium, MOL / L). After the dropwise addition, the reaction was incubated at -5 ° C for 1 hour. Then, 4.16 g (130 mmol) of elemental sulfur was added portionwise at the same temperature, and the temperature was raised for 8 hours. Thin layer chromatography was used to detect the raw material spots, and then quenched in an ice-water bath. The pH was adjusted to 6.0 to 6.5 by the addition of dilute hydrochloric acid, and then the reaction system was concentrated to dryness under pressure. The concentrated solid was dissolved in ethyl acetate and activated carbon. The reaction mixture was decolorized by refluxing for about 1 hour. The filtrate was concentrated under reduced pressure to give 9.41 g of methimazole as a pale yellow solid. The yield was 82.54% and the purity was 100% (gas chromatography).[1]
Liver Toxicity of Methimazole
Methimazole (meth im' a zole), which is also known as thiamazole, is a thioamide and a thyroid hormone antagonist which acts by inhibiting the incorporation of iodine into tyrosyl residues of thyroglobulin and, thus, lowering thyroid hormone levels. Methimazole resembles propylthiouracil both in chemical structure and activity. Methimazole was introduced into use in 1954 and is still widely used for the temporary amelioration of hyperthyroidism in Graves disease, particularly in patients with mild or self-limited hyperthyroidism or who wish to avoid thyroidectomy or radiation therapy. Because of the hepatotoxicity of propylthiouracil which can be fatal, methimazole is now considered the first line treatment for hyperthyroidism when there is a need to avoid surgery or radioiodine therapy. Methimazole is available in generic forms and under the brand name of Tapazole as tablets of 5 and 10 mg. The usual dose in adults is 15 to 60 mg daily in three divided doses until the patient is euthyroid, followed by a maintenance dose of 5 to 15 mg daily. Common side effects include gastrointestinal upset, headache, drowsiness, arthralgias, paresthesias, hair loss and rash. Rare complications of methimazole (<1%) include agranulocytosis, aplastic anemia, nephritis and hepatitis.[2]
Methimazole has been associated with transient, asymptomatic elevations in serum aminotransferase levels, typically during the first 3 months after starting during high dose, induction therapy. These elevations are rarely clinically significant and usually resolve even with continuation of therapy. Methimazole is also capable of causing clinically apparent, idiosyncratic liver injury. The onset of hepatotoxicity is usually within 2 to 12 weeks of starting and the pattern of enzyme elevations is typically cholestatic or mixed, although hepatocellular patterns have also been described. The cholestatic hepatitis caused by methimazole can be prolonged, but fatalities are rare and symptoms and jaundice usually clear within 2 to 8 weeks of stopping therapy. Rare instances of prolonged cholestasis have been described, but no instance of vanishing bile duct syndrome. Complicating the assessment of the role of methimazole or propylthiouracil in causing liver injury is the fact that hyperthyroidism by itself can cause liver test abnormalities and even jaundice. Indeed, more than half of patients with untreated hyperthyroidism have serum enzyme abnormalities (usually less than 5 times the upper limit of the normal range) and a small proportion are jaundiced and present with cholestatic hepatitis. The liver test elevations are most frequent in patients with high output heart failure. The abnormalities resolve rapidly with treatment of hyperthyroidism either with surgery, radioactive iodine or antithyroid medications.
Methimazole-induced insulin autoimmune syndrome
Hypoglycemia in a critical care setting is often multifactorial with iatrogenic insulin use, sulfonylurea (SU) use, sepsis, adrenal insufficiency and insulinoma among the common causes. Insulin autoimmune syndrome (IAS) is a rare cause of hypoglycemia characterized by the presence of insulin-binding autoantibodies to the sulfhydryl group-containing agents. We report a case of methimazole-induced IAS managed in the intensive care unit. Fluid resuscitations with normal saline and 50% dextrose stabilized the blood pressure (BP) to 135/75 and her blood glucose to 264. Due to respiratory distress and septic appearance, she required emergency intubation. Nursing home medications were noted for methimazole and absence of any insulin or SU use.[3]
Fluid resuscitations with normal saline and 50% dextrose stabilized the blood pressure (BP) to 135/75 and her blood glucose to 264. Due to respiratory distress and septic appearance, she required emergency intubation. Nursing home medications were noted for methimazole and absence of any insulin or SU use. However, it did not explain the elevated insulin levels and the recurrent hypoglycemia despite dextrose infusion and steroids. Negative SU screen, CT and a lack of response to octreotide ruled out SU ingestion and insulinoma. To our regret, IAS was a diagnosis of exclusion, since IAA levels were not collected in time. The hyperinsulinemic hypoglycemic state with methimazole use, and its resolution after 10 days of its cessation was highly suggestive of methimazole-induced IAS. IAS is associated with methimazole use due to formation of autoantibodies to insulin after its interaction with Sulfhydryl (SH) group in methimazole. While IAS is a rare entity, it demands consideration in hypoglycemia in patients with autoimmune conditions.
The efficiency and safety of methimazole in hyperthyroidism
Methimazole (MMI) and propylthiouracil (PTU) are 2 most extensively used ATDs for patients with hyperthyroidism. MMI and PTU are effective inhibitors of thyroid iodide peroxidase, which can catalyze the biosynthesis of thyroid hormone from the initial step. Although Methimazole and PTU were validated to have effects on treating hyperthyroidism, they might have adverse reactions. Previously, a study has demonstrated that PTU has a high risk of adverse reactions compared with Methimazole in the treatment of hyperthyroidism. Meanwhile, another study has suggested that PTU and MMI has a similar risk of adverse events during the treatment of hyperthyroidism. These controversial results require additional studies to make it clear about the clinical outcomes of hyperthyroidism patients after the treatment of PTU and MMI. This meta-analysis was performed to better understand the efficacy and safety of PTU and MMI in the treatment of hyperthyroidism.[4]
In this study, we found the risk of liver function damage in the Methimazole treatment group were lower than those in the PTU treatment group. Liver function damage is a pivotal adverse event of PTU and MMI treatment in hyperthyroidism patients. PTU may have higher risk of liver function damage than Methimazole. A study from Liaw et al reported that subclinical and asymptomatic liver injury can be commonly induced by PTU. Tamagno revealed that PTU treatment has a higher risk of hepatotoxicity than MMI. According to the results from the report of Russo et al, PTU ranked the third leading cause of drug-induced liver failure requiring transplants with 23 cases receiving liver transplants between 1990 and 2007 in the United States. The risk of hypothyroidism was higher in the Methimazole treatment group than those in the PTU treatment group in our meta-analysis. In previous study, 10 mg daily administration of Methimazole was found to cause spontaneous hypothyroidism in 2 patients with diffuse goiter among 36 participates. These findings implied that the clinicians might be careful with the dose of MMI in patients to avoid hypothyroidism.
References
[1]WUHAN CONFORM NEW DRUGS - CN105541724,2016, A
[2]LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Methimazole. [Updated 2020 Jan 22].
[3]Jain N, Savani M, Agarwal M, Kadaria D. Methimazole-induced insulin autoimmune syndrome. Ther Adv Endocrinol Metab. 2016 Aug;7(4):178-81.
[4]Tan S, Chen L, Jin L, Fu X. The efficiency and safety of methimazole and propylthiouracil in hyperthyroidism: A meta-analysis of randomized controlled trials. Medicine (Baltimore). 2021 Jul 30;100(30):e26707.
Lastest Price from Methimazole manufacturers

US $0.00-0.00/kg2025-10-15
- CAS:
- 60-56-0
- Min. Order:
- 1kg
- Purity:
- 98.0 ~ 101.0 %,USP43
- Supply Ability:
- 200kg/month

US $150.00/kg2025-04-21
- CAS:
- 60-56-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500kg