Mechanism and Uses of Gemifloxacin
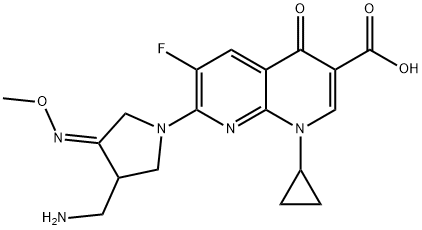
Gemifloxacin, although technically a 6-fluoronaphthyridone, is usually considered among the closely related fluoroquinolone class of antimicrobial agents. Originally designated LB20304, gemifloxacin was synthesized by LG Chemical Ltd in Korea. The chemical formula of gemifloxacin is (R,S)-7-[(4Z)-3-(aminomethyl)-4-(methoxyimino)- 1-pyrrolidinyl]-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8- naphthyridine-3-carboxylic acid. In 2003, the US Food and Drug Administration (FDA) approved the mesylate salt of gemifloxacin as an antibacterial agent for oral administration, in the form of tablets each containing the equivalent of 320 mg of gemifloxacin.

Mechanism
Fluoroquinolones act on the bacterial cell by interference with processes mediated by the two topoisomerase enzymes, DNA gyrase and topoisomerase IV. DNA gyrase mediates negative supercoiling of DNA, which is involved in DNA replication and transcription; topoisomerase IV allows decatenation of intertwined DNA strands after replication.
Fluoroquinolones stabilize the enzyme–DNA cleavage complex formed during activity of these enzymes and block replication, which is believed to ultimately result in cell death. Mutations affecting one or both subunits of these two enzymes, as well as enhanced drug efflux, contribute to fluoroquinolone resistance. Gemifloxacin inhibited the activities of purified DNA gyrase and topoisomerase IV from S. aureus to a generally similar degree. Despite this balanced effect on the purified enzymes, firststep S. aureus mutants selected on gemifloxacin-containing media had mutations in topoisomerase IV genes, indicating that this is likely the preferred target for intact cells of this species.
In contrast, with pneumococci, first-step mutants occurred in gyrA, consistent with DNA gyrase being the primary target of gemifloxacin in this species. However, first-step mutations of topoisomerase IV have also been encountered among S. pneumoniae mutants.
Uses
Gemifloxacin is available in the USA, Mexico, Korea, South Africa, and Russia. In the USA, gemifloxacin mesylate is approved for the treatment of acute bacterial exacerbations of chronic bronchitis (AECB) and community-acquired pneumonia of mild to moderate severity, proven or suspected to be caused by S. pneumoniae, H. influenzae, M. catarrhalis, K. pneumoniae, M. pneumoniae, or C. pneumoniae.
US $1.10/g2021-07-20
- CAS:
- 175463-14-6
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons Min