Luliconazole: An Antifungal Agent with High Safety Profile in Onychomycosis Treatment
General Description
Luliconazole is a highly potent antifungal agent with proven efficacy in treating fungal infections, including onychomycosis. It operates through dual mechanisms: fungicidal activity by inhibiting ergosterol biosynthesis, crucial for fungal cell membrane integrity, and fungistatic activity by suppressing extracellular protease secretion. Its effectiveness, demonstrated by lower minimum inhibitory concentrations compared to other antifungals, along with its ability to penetrate the nail plate rapidly, makes it a promising treatment for nail fungus. Additionally, Luliconazole's safety profile is commendable, displaying high tolerability and no significant adverse effects in both human and animal studies. Its rapid action and safety underscore its potential as an effective option for managing fungal infections.

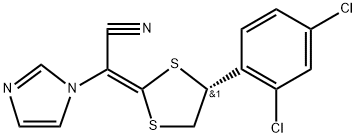
Figure 1. Luliconazole
Fungicidal and fungistatic activity
Luliconazole is a potent antifungal agent known for its dual action against fungal infections. It exhibits fungicidal activity, effectively killing fungi, by inhibiting the biosynthesis of ergosterol, a critical component of the fungal cell membrane. This action disrupts the cell membrane's integrity, leading to the death of the fungal cells. Remarkably, Luliconazole achieves this fungicidal effect at very low concentrations, with a minimum inhibitory concentration (MIC90) of just 0.001 µg/ml for Trichophyton rubrum, showcasing its high potency. Additionally, Luliconazole displays fungistatic activity, which means it can inhibit the growth and reproduction of fungi. This is achieved through the suppression of extracellular protease secretion, an important factor in fungal pathogenicity, at concentrations below those needed for its fungicidal action. Comparative studies have highlighted that Luliconazole's effectiveness, demonstrated by its lower MIC values, surpasses that of several other antifungal drugs like terbinafine, bifonazole, clotrimazole, miconazole, and amorolfine hydrochloride. This broad-spectrum activity against both dermatophyte and non-dermatophyte pathogens, confirmed through in vitro and in vivo research, underscores Luliconazole's significance as a highly effective treatment for fungal infections. 1
Clinical applications in onychomycosis
Luliconazole, with its effective antifungal properties, particularly against Trichophyton spp. and other dermatophytes, has been formulated into a 10% solution for the treatment of onychomycosis, a common nail infection. Its ability to penetrate healthy human toenails has been demonstrated in vitro, showcasing its potential for deep nail treatment. The formulation's flux rate, indicating the amount of drug delivered per unit area over time, was calculated based on luliconazole's penetration through the nail plate. This rate, along with the topical efficacy coefficient—a predictor of clinical efficacy—suggests that luliconazole can achieve therapeutic levels across the full thickness of the human nail plate within just 7 days of daily application. The efficacy coefficient, combined with a flux rate of 107.75 µg/cm^2/day, underscores the high potential for clinical effectiveness in treating onychomycosis. Further evidence of luliconazole's effectiveness comes from an in vitro study using a T. rubrum-infected nail model. The study found that the 10% luliconazole solution could rapidly cross the nail bed, eradicating the fungal load within 21 days. Achieving therapeutic levels across full-thickness human nails within a week of daily dosing further supports luliconazole's suitability as a rapid and effective treatment option for onychomycosis. This combination of rapid nail penetration and potent antifungal activity positions luliconazole as a promising candidate for onychomycosis therapy. 2
Safety and tolerability
Luliconazole has been recognized for its high safety profile and good tolerability in both single and repeated application studies on human subjects, indicating its suitability for treating fungal infections without causing significant adverse effects. Reports highlight that skin irritation indices for Luliconazole fall within the safe range, affirming its gentle nature upon application. Furthermore, studies involving guinea pig models with tinea pedis (athlete's foot) induced by T. mentagrophytes have not reported any toxicity issues, showcasing Luliconazole's safety across different species. A notable 39-week dermal toxicity study conducted on Gottingen Minipigs using a 10% Luliconazole solution demonstrated no treatment-related adverse events, even at doses up to 100 mg/kg/day. This suggests a high tolerance level for the topical application of Luliconazole. Toxicokinetic analysis further supports its safety, revealing that the area under the curve (AUC) values in minipigs at the highest dose were substantially higher than those observed in human pharmacokinetic studies, without adverse effects. Consequently, a no observed adverse effect level (NOAEL) for both systemic and local toxicity was established at 100 mg/kg/day following the prolonged application, highlighting Luliconazole's safety and tolerability for dermatological use. 2
Reference
1. Koga H, Nanjoh Y, Makimura K, Tsuboi R. In vitro antifungal activities of luliconazole, a new topical imidazole. Med Mycol. 2009;47:640-647.
2. Scher RK, Nakamura N, Tavakkol A. Luliconazole: a review of a new antifungal agent for the topical treatment of onychomycosis. Mycoses. 2014;57(7):389-393.
Related articles And Qustion
Lastest Price from Luliconazole manufacturers
US $0.00/kg2025-11-21
- CAS:
- 187164-19-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00-0.00/g2025-04-14
- CAS:
- 187164-19-8
- Min. Order:
- 100g
- Purity:
- 99%
- Supply Ability:
- 288kg