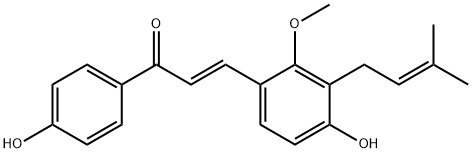
LICOCHALCONEC: A Comprehensive Exploration of Its Mechanism, Pharmacokinetics, and Clinical Considerations
Licochalcone C is a yellow crystalline powder derived from licorice rhizomes.

Mechanism and pharmacokinetics
The current study investigated the mechanisms by which licochalcone C induces apoptosis of T24 human malignant bladder cancer cells. Cell viability was evaluated using an MTT assay. Apoptosis was investigated using a morphological assay, flow cytometry and a caspase‑3 activity assay. Alterations in the gene expression levels of Bcl‑2 family members were measured by semi‑quantitative reverse transcription‑polymerase chain reaction assays. The protein levels of pro‑caspase‑3 and cleaved poly(ADP ribose) polymerase were measured using western blotting. The results indicated that licochalcone C induced T24 cell apoptosis in a concentration‑dependent manner. Licochalcone C treatment reduced the levels of the anti‑apoptotic mRNAs (Bcl‑2, Bcl‑w and Bcl‑XL) and increased expression of the pro‑apoptotic mRNAs (Bax and Bim). The Bcl‑2 family inhibitor (ABT‑737) reduced apoptosis induced by licochalcone C in T24 cells. The current study demonstrated that licochalcone C may be a potential adjuvant therapeutic agent for bladder cancer.1
Clinical Considerations
Licochalcone C has anti-ulcer, antibacterial and anti-inflammatory, lipid-lowering, free radical scavenging and antioxidant effects.
Synthesis
A novel efficient method for the synthesis of licochalcone C in good yield on up to 30 g scale was developed. The reaction sequence included relied on the directed ortho-metalation (DOM) of bis-O-MOM-protected resorcinol for the regioselective C-prenylation, followed by metalation-formylation, selective O-deprotection of a hydroxyl group located between the formyl and prenyl groups, its methylation, and aldol reaction with p-hydroxyacetophenone. Synthesis of structurally related retrochalcones, i.e., licoagrochalcones B, C, and D, was also proposed.
References
1.Penglong Wang.“Licochalcone C induces apoptosis via B-cell lymphoma 2 family proteins in T24 cells.”Molecular medicine reports 12 5 (2015): 7623–8.
You may like
Related articles And Qustion
Lastest Price from LICOCHALCONEC manufacturers

US $0.00/kg2024-04-12
- CAS:
- 144506-14-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000ton
US $0.00/mg2023-02-24
- CAS:
- 144506-14-9
- Min. Order:
- 10mg
- Purity:
- ≥98%(HPLC)
- Supply Ability:
- 10 g