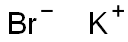Lewis structure of Potassium bromide
Potassium bromide (KBr) is an inorganic ionic compound. Unlike covalent (molecular) compounds, for ionic compounds, when a metal is bonded to a nonmetal (or a group of nonmetals), the Lewis structure diagram represents a molecular formula unit. The Lewis structure of KBr is composed of a potassium positive (K+) ion and a bromine negative ion (Br+), where the bromine ion carries 4 lone pairs of electrons, and the two ions are surrounded by "[ ]". This is shown in the diagram below:

The steps to draw the Lewis structure of KBr are as follows:
1. Calculate the number of valence electrons
According to the periodic table, K and Br atoms carry 1 and 7 valence electrons respectively, so the total number of valence electrons in the KBr ion = the number of valence electrons provided by 1 potassium atom + the number of valence electrons provided by 1 bromine atom = 1 + 7 = 8.
2. Determine the central atom
In metal ionic compounds, most of them use metal atoms as central atoms, so here K atom is used as the central atom.
3. Mark the lone pairs of electrons between atoms
The total number of valence electron pairs is obtained by dividing the number of valence electrons by 2. Therefore, the total number of valence electrons of KBr is 4.
4. Make the structure more stable
We know that the stability of the structure of a compound is related to its number of valence electrons. Therefore, in order to achieve the eight-atom rule, we transfer one valence electron from the potassium atom to the bromine atom to achieve a stable structure of the bromine atom. The potassium atom loses one valence electron and therefore carries a "+" charge, while the bromine atom gains one valence electron and carries a "-" charge.
Related articles And Qustion
Lastest Price from Potassium bromide manufacturers

US $1.00/KG2025-04-21
- CAS:
- 7758-02-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $6.00/kg2025-04-21
- CAS:
- 7758-02-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month