Lewis Structure, Hybridization and Polarity of Carbon Tetrafluoride
Carbon tetrafluoride is colorless and odorless gas with the chemical formula CF4. It is non-flammable gas and is used as a refrigerant in various industries. It is very stable due to the strength of its carbon-fluorine bond. The stable properties are determined by its structure.
Lewis Structure
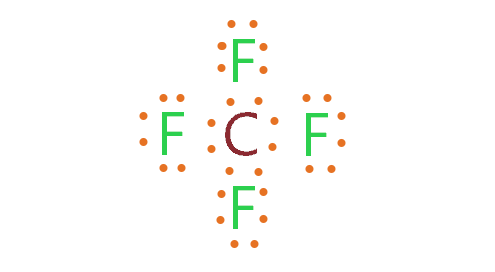
CF4 lewis structure contains one carbon and four fluorine atom, carbon is in the center, and all fluorine atoms surround it. No lone pair is present on the central atom of the CF4 lewis dot structure but 3 lone pairs are present on each outer atom.
Hybridization
Hybridization number of CF4 = (Number of atoms attached to Carbon + Lone pair on Carbon)
As per the CF4 lewis structure, carbon has four atoms(fluorine) attached to it and no lone pair present on it.
So, Hybridization number = 4 + 0 = 4
The hybridization number for CF4 is 4 which means it has Sp³ hybridization.
Polarity
Carbon tetrafluoride (CF4) has a central carbon atom surrounded by four fluorine (F) atoms. Carbon has four valence electrons, and fluorine has seven. Hence, carbon requires four more electrons, and fluorine requires one electron to complete its octet. Therefore, carbon bonds with four fluorine atoms through single covalent bonds, resulting in a tetragonal structure. In such a structure, all bonds are equidistant with a bond angle of 109.5°.
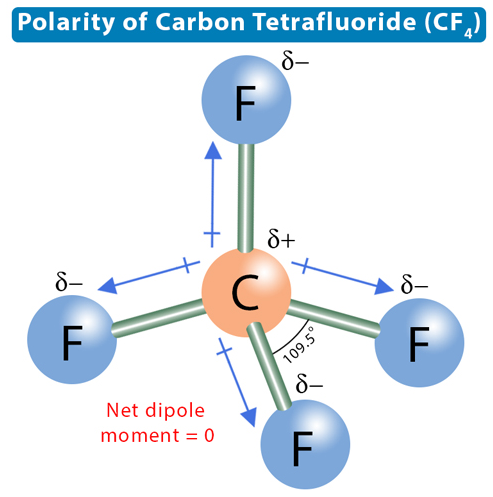
The electronegativity of carbon is 2.55, and that of fluorine is 3.98. Carbon is the least electronegative atom and sits at the molecule’s center. Fluorine is the most electronegative atom and attracts the shared electron pair toward itself. The electronegative difference between the two atoms and the ability of fluorine to attract bonding electron pairs make the C-F bond polar. There will be a dipole moment directed from C to F. However, the dipole moments cancel due to tetragonal symmetry, making CF4 nonpolar.